A 39-year-old female, liver transplanted for Autosomic Dominant Polycystic Kidney Disease (ADPKD) developed refractory ascites early after surgery, with frequent need of large-volume paracentesis. This was associated with severe sarcopenia and kidney impairment. Liver biopsy showed a sinusoidal congestion with a significant enlargement of hepatic portal veins. This picture suggested the diagnosis of vascular obstructions. Due to an unfavorable passage through the piggy-back surgical anastomosis and the angle between the hepatic veins and the portal branches, a conventional placement of a transjugular portosystemic shunt (TIPS) was not feasible. An alternative approach was pursued with success, using a combined percutaneous-transjugular approach and achieving a complete recovery of ascites, sarcopenia and renal function.
Placement of a transjugular intrahepatic portosystemic shunt (TIPS) is a minimally invasive procedure for the treatment of the major complications of portal hypertension [1–6]. The conventional procedure contemplates the catheterization of the right or the middle hepatic vein (HV) through a retrograde transjugular approach from the inferior vena cava (IVC), and the subsequent placement of a metallic covered stent, through the liver to reach the portal vein. However, in rare cases, the HVs cannot be catheterized employing a retrograde approach [7], thus other techniques may be needed to complete the procedure. TIPS placement after liver transplantation (LT) can be more challenging due to the anatomical changes of the HVs. Specific technical difficulties can be encountered when a piggyback venous anastomosis has been performed [8,9].
Herein, we describe the case of a young woman who underwent LT for Autosomal Dominant Polycystic Kidney Disease (ADPKD). This affection is one of the most frequent hereditary disorder and an important cause of kidney failure [10]. Extra-renal involvement regards the liver in the majority of cases and sometimes may evolve to complications (chronic unremitting pain, high-grade mechanical compression of vascular and/or biliary structures) requiring liver resection or transplantation [11]. Our patient, due to a reduced venous outflow through the piggyback anastomosis, developed early after transplant portal hypertension with refractory ascites. This was resolved by an unconventional placement of TIPS through a combined percutaneous-transjugular approach.
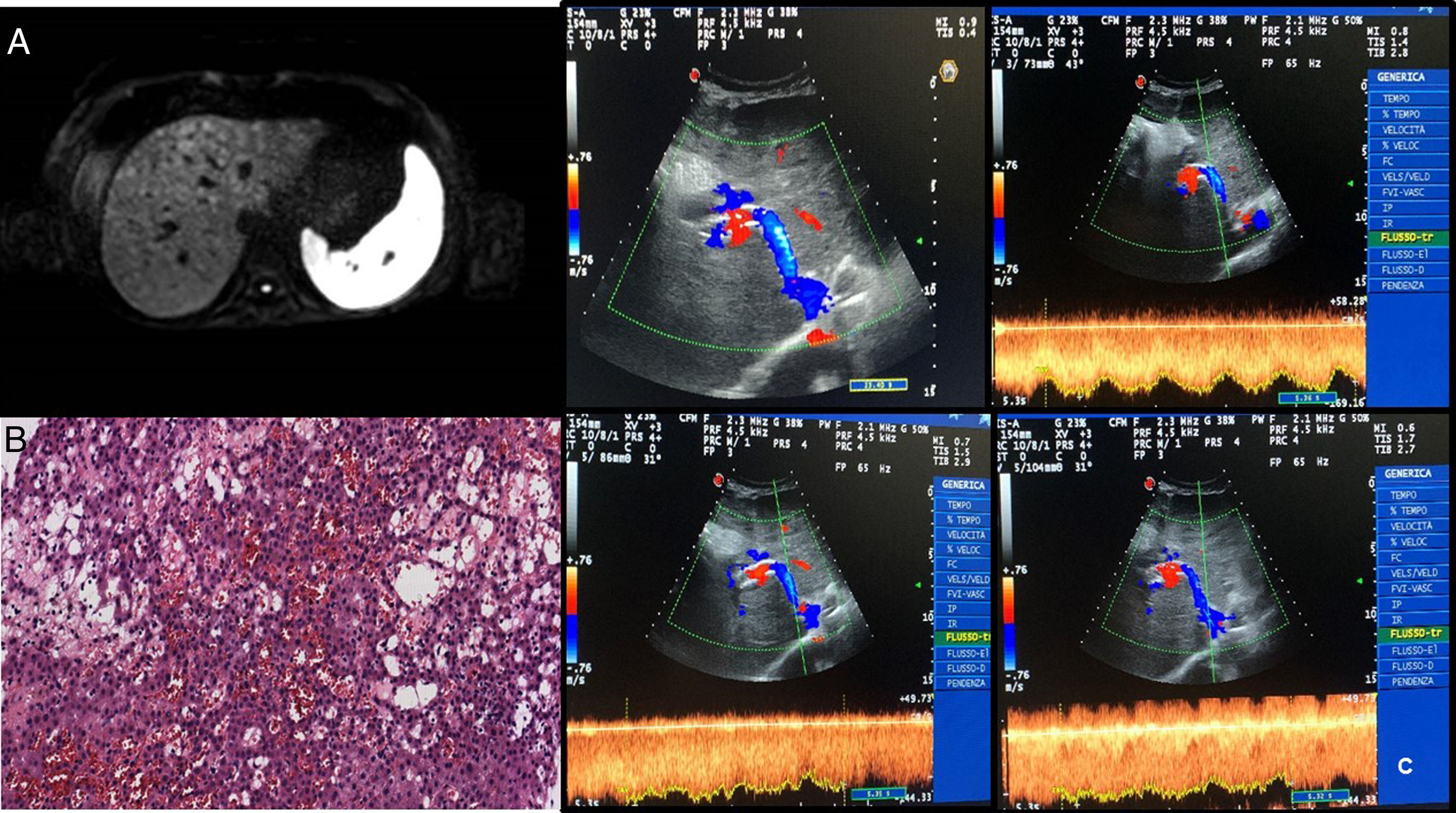
2Case reportA 39-year-old female underwent LT (Sept. 2017) for ADPKD. The postoperative course was characterized by a persistent rise of liver function tests, with signs of cytolysis and cholestasis, as well as by the appearance of ascites. High dose of furosemide and spironolactone (75mg and 400mg, respectively) resulted in poor efficacy. In May 2018, blood tests showed a slight reduction of platelet count and the deterioration of kidney function (creatinine 3.19mg/dl), associated with chronic diarrhea. Moreover, the patient significantly reduced her food intake because of dyspeptic symptoms. As a result, she developed severe sarcopenia (L3SMI 32cm2/m2; brachial circumference 20cm). The patient was hospitalized and multiple large volume paracentisis (LVP) were performed. Analysis of ascitic fluid revealed an albumin concentration of 0.7g/dL with a serum-ascites albumin gradient>1.1 suggesting portal hypertension. No signs of spontaneous bacterial peritonitis were detected. A contrasted-enhanced computed-tomography (CT) scan showed patency of portal vein, hepatic artery, and piggyback anastomosis. Upper gastrointestinal endoscopy did not show esophageal or gastric varices. A contrast nuclear magnetic resonance (NMR) study revealed multiple hypodense small lesions (<2cm diameter), more evident at the dome and the right lobe, with shrinking of the signal in Diffusion Weighted Imaging sequences. A minimal dilatation of the intrahepatic bile ducts was also observed (Fig. 1, Panel A).
(A) Post-contrastographic sequences of liver MRI showing multiple hypodense focal lesions, more evident at the dome and the right lobe which show shrinking of the signal in DWI sequences; concomitant minimal dilatation of the intrahepatic bile ducts. (B) Section of hepatic specimen (1921×991; 20×) with evidence of hematologic extravasation, hepatocyte necrosis, focal dilation and congestion of sinusoids. (C) 6-Months after TIPS placement Doppler examination showing the patency of intrahepatic stent with high caval and portal velocity; no evidence of abdominal ascites.
A measurement of the Hepatic Venous Pressure Gradient was planned in order to evaluate the potential benefit of TIPS placement. Moreover, a liver biopsy was performed, showing focal sinusoid dilation and congestion with loss of endothelial lining. In some fields, dilation of the centrilobular veins was also observed (Fig. 1, Panel B).
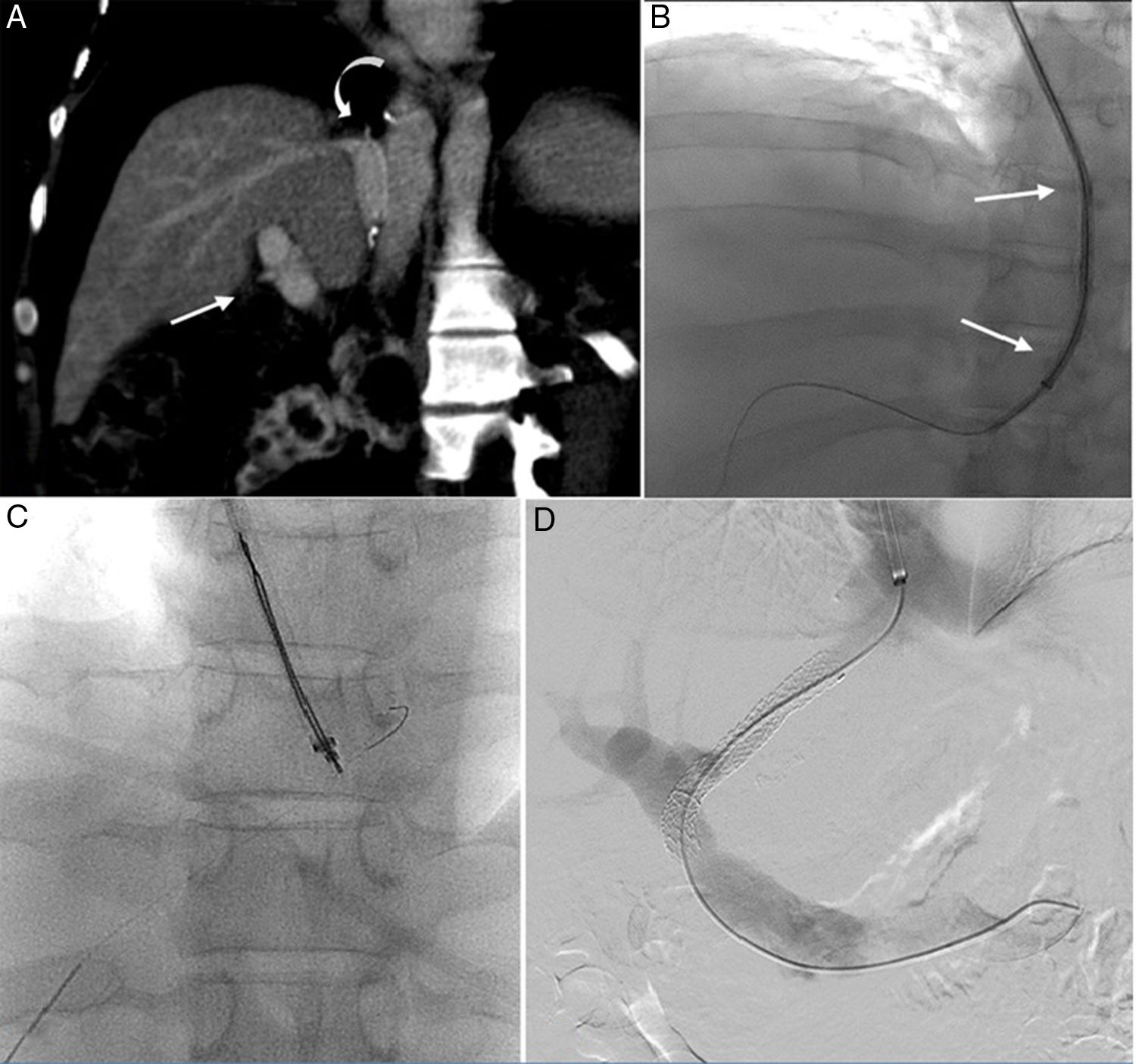
Although the morphological picture was unspecific, the sinusoid damage was suspected to be related to vascular obstruction. Based on the latter, placement of TIPS was decided. The first attempt, based on conventional transjugular approaches (Fig. 2, Panel A), failed because of the anatomical complexity. The surgical anastomosis formed an acute angle between the HVs and the portal branches. This, in turn, resulted in portal hypertension.
(A) CT scan showing the caudal localization of surgical anastomosis (curved arrow) and the subsequent acute angle between the hepatic vein and the portal branches (straight arrow). (B) Unsuccessful attempt at shaping 14 G stiffening cannula (straight arrow); the procedure was interrupted because only the puncture of the extrahepatic segment of the main portal vein was obtained. (C) Puncture of the left portal vein branch and the hepatic vein directly in a single pass; placement of a 300cm long 0.0014 guidewire from the hepatic vein, through the inferior vena cava and into the previously placed trans-jugular sheath. (D) Final portal phlebography showing the TIPS placement.
A second attempt was made using a combined percutaneous-transjugular approach (Fig. 2, Panels B and C). First, a percutaneous puncture of the left portal vein branch and the HV directly, in a single pass, using a 21-gauge needle under ultrasound guidance, was performed. A 300cm long 0.0014’ guidewire was placed from the HV, through the IVC, and into a previously placed transjugular sheath, thus achieving through-and-through access. The advancement of various devices from the transjugular access was then easily obtained despite the acute angle, pulling the two extremities of the guidewire. A 4F vertebral diagnostic catheter was advanced from the transjugular access to the left portal branch and a second 0.014’ guidewire was advanced through the main portal vein and into the splenic vein. The diagnostic catheter was removed and a dedicated below-the-knee 5mm×60mm monorail balloon catheter was advanced over the second guidewire and inflated. Then, the jugular sheath was advanced into the portal branches. Once the introducer sheath was located in the portal system, a 260cm long 0.035-in. superstiff guidewire was placed into the splenic vein, and finally, the 0.014’ guidewire was removed from the two percutaneous accesses. The stent system (10mm×80mm, Viatorr, Gore) was introduced via a transjugular shunt over the working wire, placed, and post-dilated with a 10mm diameter angioplasty balloon catheter (Fig. 2, Panel D).
The post-procedure phase was uneventful. After six months, the patient showed a six-centimeter increase of the brachial circumference and gained five kilograms in weight. There was no evidence of ascites and blood test was significantly improved, including renal function tests, with complete recovery of normal serum creatinine levels and platelets count. The TIPS was performing well (Fig. 1, Panel C). Regardless of the TIPS placement, the patient did not experience any encephalopathy during follow-up. This was probably related to a well preserved liver function despite the presence of the venous obstruction in the previous months.
3DiscussionRefractory ascites (RA) is an infrequent, yet serious, complication after LT, with an incidence of about 5.9% and characterized by a poor prognosis [12]. Among the vascular causes of RA in this setting, stenosis, splenic arterial steal syndrome, and left-sided portal hypertension are included. However, venous outflow complications are relatively uncommon [9]. Acute manifestations with liver failure are more frequent in pediatric patients and often due to technical problems, such as a too-tight anastomosis. A delayed presentation, usually deriving from perivascular fibrosis or intimal hyperplasia, is more common among adults. These conditions cause graft edema and hepatic enlargement, thus resulting in obstruction of the caval and HV outflow due to extrinsic compression of the IVC [13]. The hydrodynamic change explains the typical presentation with RA, pleural effusion, and increase of liver function tests, in these subjects. In our case, there was also an elevation in serum creatinine and alterations in tacrolimus metabolism. These are both a common manifestation of HV outflow obstruction [9]. The diagnosis is frequently obtained by CT or NMR scan. In regard to vascular stenosis, several techniques gave good result and reproducibility such as color Doppler ultrasound [14], computed tomography angiography [15] or also Time resolved imaging of contrast kinetics NMR angiography [16]. In addition, a selective catheterization of the HV is of value to confirm the stenosis and the increase in the pressure gradient. In fact, up to now, venography and pressure gradient measurements still represent the diagnostic gold standard [8,9].
TIPS role, in the treatment of ascites after orthotopic liver transplant, is reported to have variable success (16–57%) [17] and technical difficulties can be encountered when the piggyback anastomosis is performed [8,9]. In these cases, alternative approaches for a TIPS placement are considered using transcaval [13,17] or pull-through [17] techniques.
In our case, TIPS placement allowed the complete resolution of the patient's clinical picture, making a drastic advantage in term of quality of life. The hypothesis is that its presence helped resolve the sinusoidal damage and, as the distal part of the device ended over the HV, it widened the venous anastomosis permitting better blood flow. As it was not possible to perform the TIPS placement through the conventional way, we performed for the first time a combined transjugular-percutaneous approach that provided a competitive and reproducible procedure. In addition, while diagnosis and treatment of venous outflow obstruction has been described in patients with polycystic liver disease, we report the first description of this occurrence, after transplant, in this category of patient.AbbreviationsADPKD Autosomic Dominant Polycystic Kidney Disease hepatic vein inferior vena cava liver trasplantation large volume paracentisis computed-tomography nuclear magnetic resonance refractory ascites
None.
Conflict of interestNone.