Liver transplantation is regarded as an effective treatment for Wilson’s disease (WD), and recently has been shown to improve not only hepatic but also neurologic manifestations. Conventional auxiliary liver transplantation for WD is orthotopic liver transplantation and heterotopic liver transplantation. But the conventional procedure could not avoid the problem of space, functional competition, hemodynamic variation. Here we report a case of heterotopic auxiliary living-donor liver transplantation (HALDLT) to treat WD. We modified the operation to have a splenectomy, implant graft into the splenic fossa. The patient recovered well after the transplantation and has been symptom-free during a 5-year follow-up. This modified operation is more safe and simple. HALDLT might be an effective treatment for WD patients with splenomegaly.
Liver transplantation (LT) in the pediatric population has become a viable treatment method for children with end-stage liver disease.
Technical advances in the transplantation procedure such as auxiliary liver transplantation, reduced-size transplantation, split liver transplantation, and use of living donors for partial liver transplantation,1,2 as well as modifications to immunosuppression and improvements in prophylaxis, have led to better survival rates in pediatric patients.
Wilson’s disease (WD) is an autosomal, recessive, inherited disorder of hepatic copper metabolism that results in the accumulation of copper in many organs and tissues. The hallmarks of the disease are the presence of liver disease, neurological symptoms and Kayser-Fleischer corneal rings.
LT is regarded as an effective treatment for WD, and recently has been shown to improve not only hepatic but also neurologic manifestations.3–5 Stampfl, et al. reported a successful heterotopic auxiliary partial liver transplantation in a patient (HAPLT) with Wilson’s disease presenting with fulminant hepatocellular failure. The advantages of HAPLT include avoidance of the anhepatic phase, limited surgical trauma, and less operating room time with elimination of recipient hepatectomy.6 Here we report a case of heterotopic auxiliary living donor liver transplantation (HALDLT) to treat WD using a novel operation procedure and producing a good recovery.
Case ReportIn June 2007, a 12-year-old girl with Wilson’s disease, presenting with manifestations including chronic liver failure, tremors and dyskinesia, underwent HALDLT in our unit. At the time of writing, the patient is alive and well with persistent normalization of graft function for 5 years.
Before transplantationThe patient was diagnosed with Wilson’s disease in 2006 after her parents noticed mild tremors, speech and writing problems, and bilateral leg edema. In March 2007, she developed progressive movement disorder characterized by dysarthria, apraxia and a tremor-rigidity syndrome (also known as ‘juvenile Parkinsonism’).7 Her liver dysfunction gradually deteriorated into decompensated liver cirrhosis. Laboratory evaluations showed hypoalbuminemia (32 g/L), leukopenia (2.1 x 109 cells/ L), thrombocytopenia (39 x 109/L), and high unconjugated bilirubin (23 μmol/L). Prothrombin time (PT) was 16.1 s.
The patient was 140 cm tall and 30.0 kg weight when she is on admission. Vital signs were within normal limits. Physical examination on admission showed splenomegaly and mild ascites. Abdominal ultrasonography and computed tomography revealed marked liver cirrhosis and splenomegaly.
Copper metabolism was evaluated in our laboratory. The results showed a low plasma copper level (1.0 μg/dL) and a low plasma ceruloplasmin level (2.0 mg/dL). Her 24-h urine copper excretion was normal because she had been receiving continuous anti-copper therapy (D-penicillamine and zinc sulfate). Kayser-Fleischer (KF) rings were found by slit lamp examination. T2-weighted magnetic resonance imaging (MRI) revealed bilateral lucencies in the basal ganglia and thalami.
HALDLT was performed in June 2007. The donor was her mother who was heterozygous for the Wilson genetic defect. She had no history suggestive of WD, and her serum copper and ceruloplasmin levels were 3.4 μg/dL and 20.2 mg/dL, respectively.
Donor surgical procedureThe donor was evaluated according to a previously reported algorithm including donor liver biopsy and hepatic angiography.8 Histological examination of the liver showed moderate infiltration by aggregates of lymphoid cells as well as mild piecemeal necrosis. Size calculations for this recipient and her liver graft were performed using 3-dimensional computerized tomographic imaging. Volumetric study estimated that the left lobe volume was 255.98 cm3.
Left hepatectomy (segment 2, 3 and 4) were performed with an ultrasonic dissector and bipolar electrocoagulation. Routine intraoperative cholangiography was performed before bile duct splitting. On the back table, the liver graft was flushed with University of Wisconsin (UW) solution. The diameters of the left portal vein, left hepatic artery and vein of the graft were 6, 2, and 14 mm respectively. The graft to recipient body weight ratio was 0.77%.9
Recipient surgical procedureThe graft was performed using the following operation procedure:
- •
Leave the native liver intact.
- •
Graft the implant into the splenic fossa.
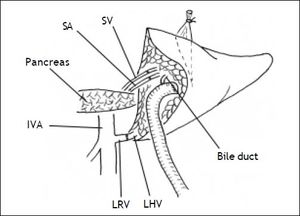
Firstly, a complete splenectomy to make a room for the graft in the splenic fossa was performed. Before the resection, the splenic vein and artery were identified since they passed above the body of pancreas. Close to the hilum of the spleen, these two vessels were divided and ligated. Then, the tail of the pancreas was isolated, thereby creating a small recess anterior to the left renal vein. Following this, the left renal vein was divided and ligated. In order to retain the left gonadal vein and the central vein of the suprarenal gland, we isolated the left renal vein as closely to the vena cava as possible to allow application of a Satinsky vascular clamp. The diameters of the recipient’s splenic vein, splenic artery and left renal vein were carefully measured (7, 2, 13 mm respectively). Splenic venous pressure was 24 and 19 mmHg before and after spleentecomy.
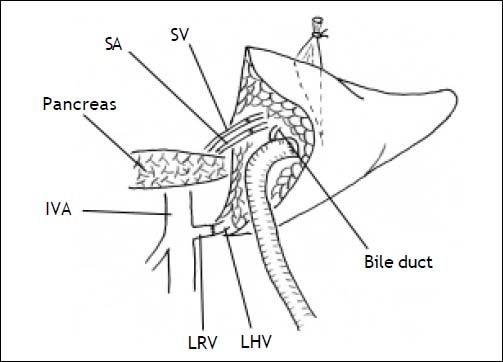
Secondly, the graft was implanted heterotopically into the splenic fossa. The left hepatic vein of the graft and the free margin of the recipient left renal vein were brought together. A continuous running suture using a 4-0 Prolene stitch (Ethicon, Sommerville, NJ) was placed between these two veins in an end-to-end fashion. Then, the graft portal vein was anastomosed with the recipient’s splenic vein in an end-to-end fashion. Reconstruction of the left hepatic artery and splenic artery was performed with a corner-saving suture (9-0 silk suture thread, Ethicon), end-to-end anastomosis using loop magnification. Intraoperative Doppler ultrasound examination showed that all vessels were patent. An anastomosis between the left bile duct and a Roux-en-Y jejunum loop restored the bile drainage (Figure 1). A thin polyethylene catheter was inserted through the choledochojejunostomy into the Roux-en-Y limb and attached to a collecting bag via a stab wound in the abdominal wall for bile production monitoring.
Immunosuppressive therapyPost operation, the patient received well-described immunosuppressive protocol.10,11 Intravenous methylprednisolone (10 mg/kg) administered on the day of transplantation. It was continued postoperatively at dosages tapered from 10 mg/kg to 0.1 mg/kg at the end of the first month. A maintenance prednisone dose of 0.25 mg/kg/d was continued for 1 month, after which time steroid therapy was stopped. Tacrolimus (FK506, Prograf, Astellas) was used for maintenance therapy. The maintenance dosage of tacrolimus was adjusted to maintain a level of 8 to 10 ng/mL during the first 2 months and 7 to 8 ng/mL thereafter.
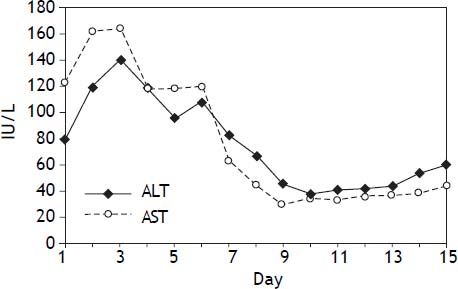
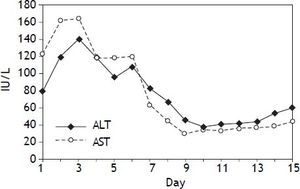
Liver function and renal function monitorAfter operation, blood samples were obtained from peripheral veins at 6:00 and 12:00 daily to measure the levels of serum aspartate aminotransferase, alanine transaminase, bilirubin, serum copper and ceruloplasmin. One week later, serum aspartate aminotransferase and alanine transaminase reached normal levels (Figure 2).
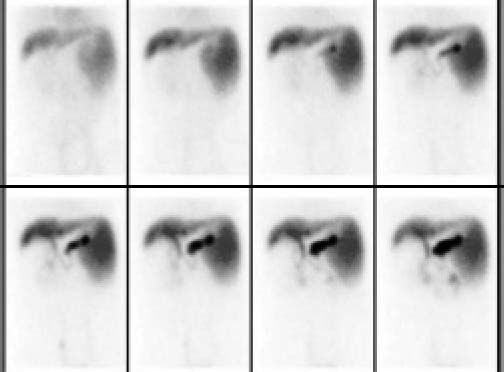
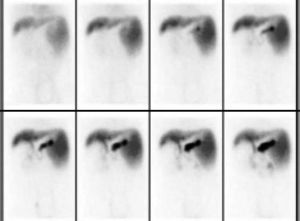
The graft and native liver were observed by 99mTC-Sodium phytate. The result showed that the function of the graft was well (Figure 3).
Although the blood urea nitrogen (BUN) and creatinine were not influenced by the ligation of left renal vein, the red blood cell in urine was observed after several days postoperation. Haematuria disappeared as soon as the size of left kidney recovered to normal on the 7th day post operation.
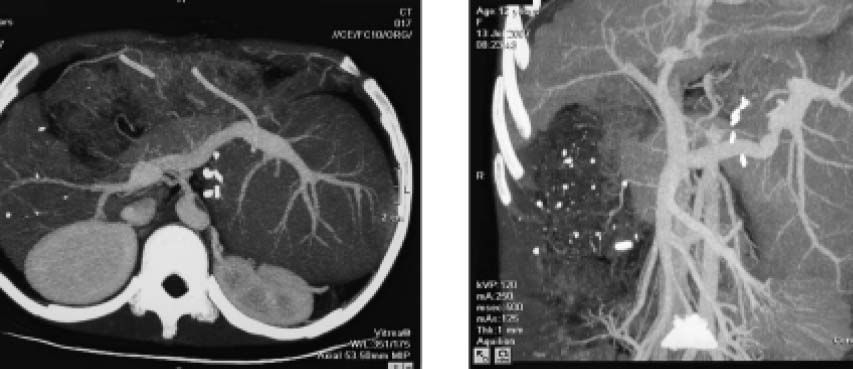
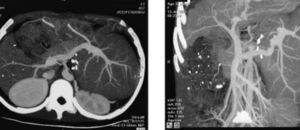
One month after HALDLT, the recipient’s serum copper and ceruloplasmin reached normal levels. Neurological symptoms also dramatically improved. CT showed that the graft had proliferated significantly at the same time (Figure 4). Two months later, neurological symptoms became almost normal. Liver biopsy in the heterograft eight months after transplantation showed the structure of hepatic lobule was normal with Local hepatic Sinus Dilation, a few cell degeneration and necrosis could be found in central lobular area, as well as a few lymphoid cells infiltration in portal area.
Doppler ultrasoundBy using Doppler ultrasound, we were able to observe a good spectrum of the waveform of the graft, which demonstrated that the graft was receiving a better blood supply according to the formula.12 Doppler ultrasound exam showed that the portal vein flow velocity was 60 cm/s 2 days after liver transplantation. The left portal vein flow velocity decreased to 40 cm/s 2 months after transplantation, when the blood flow in portal vein of the native liver was so poor that sometimes it could not be detected.
At the time of writing, both the recipient and the donor were leading normal lives, and the recipient was free of neurological symptoms.
DiscussionSince the first successful liver transplantation for WD in 1971,13 a number of reports in the literature have considered WD as an excellent indication for transplantation, which can abrogate biochemical and clinical signs and offer long-term survival.14,15
Previous studies have never implanted the graft in the splenic fossa to solve the “space problem”. To date, there are few reports of living donor liver transplantation (LDLT) for WD. Asonuma, et al.16 described 11 pediatric (ages 6-16 yrs) cases of LDLT in which all liver grafts were obtained from parents who were one-haplotype matched and naturally heterozygous for the Wilsonian genetic defect. They reported that all recipients had improved copper metabolism and remained free of recurrent WD after transplantation in their series. They concluded that the use of partial liver grafts obtained from asymptomatic heterozygote carriers of the WD genetic defect did not pose any difficulties regarding restoration of copper metabolism. Similar results reported by Wang, et al.17 also confirmed the validity of partial liver grafts obtained from living haplo-type-matched donors.
Since the development of auxiliary partial orthotopic liver transplantation (APOLT), controversies still remain, such as the “functional competition” that results from the portal blood flow shared between the graft and the native liver. To overcome this problem, and to optimize flow to the graft, some authors have banded or even ligated the native liver’s portal vein.18 In this case, portal blood flow competition was not observed. We speculated that this was because the severe cirrhosis caused the blood flow to the graft to be more than that to native liver, which was able to be monitored by type-B ultrasound as soon as the graft was implanted. Schleimer, et al.19 reported that native liver regeneration seemed negatively influenced by a graft. These results do not support the hypothesis of functional portal flow competition between different livers. If portal inflow to the graft is decreased, some authors recommend step-by-step percutaneous embolization of the branches of the native right lateral portal vein, along with careful follow-up studies of the liver function.20
In this case, we supposed that the survival of the graft would be better if the pressure of the outflow drainage is low enough. The renal vein can provide a low outflow pressure because of its close proximity to the right atrium beside the hepatic vein. Notably, the right renal venous collateral circulation was weaker than that of the left. Choosing the right renal vein as the outflow drainage will damage the right renal function. Therefore, the left renal vein was chosen to be the outflow for the graft. Ligation of the left renal vein did not influence the renal function; in fact, the size of left renal was normal by the 7th day after operation.
In patients who have undergone LT, hypersplenism becomes problematic when persistent neutropenia and thrombocytopenia interfere with the ability to start or continue antiviral therapy or chemotherapy. Splenectomy is effective measure in liver transplant recipients for treatment of a variety of indications, including hypersplenism, prevention of gastroesophageal variceal hemorrhage, and for controlling portal pressure during small grafts in living donor liver transplantation. Splenectomy improved liver function in patients with liver cir-rhosis, and could be a supportive and bridging therapy for patients waiting for liver transplantation.21 Recent advances in surgical techniques have enabled surgeons to perform splenectomy more safely and less invasively, but the procedure still has considerable clinical outcomes. The major drawback of splenectomy is sepsis.
The potential risk for carcinogenicity of the remnant native liver is the one shortcoming of HAPLT for cirrhotic liver disease. This problem remains to be discussed. However, Kasahara’s report from the Kyoto group with more than 5 years follow-up showed no tumor development in the remnant native liver.18 Therefore, delayed native hepatectomy after complete graft regeneration might not be necessary.
Abbreviations- •
ALT: auxiliary liver transplantation.
- •
APOLT: auxiliary partial orthotopic liver transplantation.
- •
HALDLT: heterotopic auxiliary living donor liver transplantation.
- •
HAPLT: heterotopic auxiliary partial liver transplantation in a patient.
- •
LDLT: living donor liver transplantation.
- •
LT: liver transplantation.
- •
WD: Wilson’s disease.