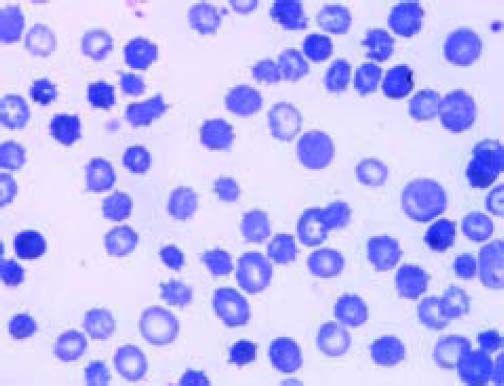
A 60-year-old patient with a history of alcoholic cirrhosis (Child-Pugh score C), diabetes mellitus and ongoing alcohol intake presented to the emergency departed with hypoxemic dyspnoea in the presence of ascites and peripheral edema. Laboratory data indicated acute renal insufficiency with preserved natriuresis (creatinine 3.7 mg/dL, normal 0.7-1.2; urea 117 mg/dL, normal 10-50; 24 h urine sodium 164 mmol/L, normal 30-300). In the absence of protein and erythrocytes in urine, this was, retrospectively, considered to be due to decompensated portopulmonary hypertension (right ventricular systolic pressure > 50 mmHg after establishing euvolemia) and responded favourably to diuretic treatment. Bidirectional endoscopy yielded grade I esophageal varices and portal gastropathy without signs of bleeding. International normalized ratio was elevated to 1.85 and serum bilirubin was 25.8 mg/dL (normal < 1.2; direct bilirubin 8.8 g mg/dL, normal < 0.3). Aspartate aminotransferase was moderately raised to 93 U/L (normal 10-50), while alanine aminotransferase, gamma-glutamyltransferase and alkaline phosphatase activities were within normal ranges as were leukocyte count and C-reactive protein. Erythrocyte-related markers were as follows: hemoglobin 6.1 g/dL (normal 14-18), hematocrit 18% (normal 41-55), mean corpuscular hemoglobin 36 pg (normal 27-33) and mean corpuscular volume 106 fL (normal 80-99). Reticulocyte count and lactate dehydrogenase were raised with 5.5 % (normal < 1.5) and 836 U/L (normal 0-262), respectively. Of note, haptoglobin was below the detection limit, but represents an unreliable hemolysis marker in advanced cirrhosis. Yet, while Coombs test was negative, a diagnosis of chronic hemolysis was unequivocally established by detection of free hemoglobin after gentle venipuncture. Thus, a peripheral blood smear was ordered and demonstrated marked poikilocytosis with abundant atypical erythrocytes with multiple spiculae-like projections, consistent with the diagnosis of advanced spur cell anemia (SCA) in the setting of decompensated alcoholic cirrhosis (Figure 1).
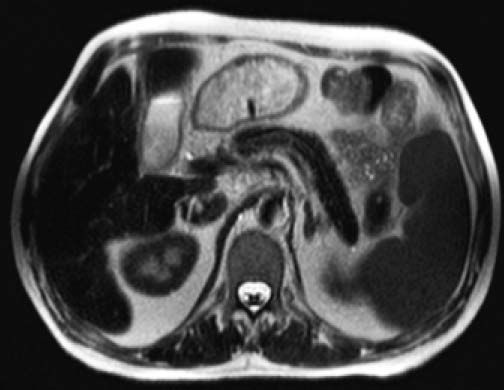
Given markedly raised ferritin levels (4,683 ng/ mL, normal 30-400) and transferrin saturation (99%, normal 16-45), genotyping of the hemochromatosis (HFE) gene was performed, and yielded a heterozygous state for the mutation p.C282Y, thus genetically excluding a diagnosis of classic hemochromatosis. However, with respect to iron status markers it has to be noted that the patient had received > 5 units of packed erythrocytes on an outpatient basis elsewhere before, complicated by the occurrence of irregular anti-E antibodies. In the presence of moderately elevated alpha-fetoprotein levels (8.3 IU/mL, normal < 5.8), the patient underwent MR imaging of the upper abdomen demonstrating a diffuse hypointense signal intensity on T2-weighted sequences of the liver, the pancreas and less pronounced of the spleen. This hypointensity of liver and pancreas parenchyma is a typical finding of iron overload due to hemochromatosis, whereas the hypointense signal of the spleen indicates increased iron accumulation in the reticuloendothelial systems, such as in hemosiderosis (Figure 2). Mixed-pattern iron depositions are uncommon, and imply an advanced form of iron excess related to chronic hemolysis and high transfusion demands in the presented patient.7
Axial T2-weighed abdominal magnetic resonance image (MRI) at the level of pancreatic body and tail. Note the homogeneous hypointense signal of the liver and pancreas indicative of diffuse parenchymal iron overload as in primary hemochromatosis. Likewise, but less pronounced, the spleen displays a hypointense signal, consistent with reticuloendothelial iron deposition, thus giving rise to a mixed-pattern of iron overload.
Due to lack of alcohol abstinence the patient was not considered suitable for orthotopic liver transplantation. He was further treated symptomatically, while remaining dependent on repeated erythrocyte transfusions, and succumbed five months later due to bacterial sepsis with purpura fulminans, attesting to the excess risk of bacterial and fungal infections in iron overload states.
DiscussionSCA represents an underdiagnosed, though common, complication of end-stage, mostly alcoholic cirrhosis, resulting in chronic hemolysis due to acquired disturbances in erythrocyte membrane physiology related to the chronic liver disease and ongoing alcohol consumption, such as imbalances of the cholesterol/phospholipid composition.1 Consecutively, red blood cell survival is shortened owing to splenic sequestration and destruction. In a recent study 31% of patients with advanced cirrhosis (Child-Pugh scores > 7) were reported to suffer from SCA, as per 5% SC rate on peripheral blood smear, which was identified as an independent predictor of early mortality in this population (hazard ratio = 3.2; 95 % confidence interval 1.6-6.5).2 Similar data were reported by Vassiliadis et al., indicating that patients with SCA have higher Child-Pugh and Model of End-stage Liver Disease (MELD) scores and a significantly reduced 3-month survival rate.3
Though there are no definitive data available for SCA and the likely role of hepcidin, intestinal iron absorption is known to be enhanced in hemolytic states.4 Of note, up to 10 % of patients undergoing orthotopic liver transplantation exhibit levels of hepatic iron overload in the range of that seen in hereditary hemochromatosis, as defined by hepatic iron index > 1.9 in the absence of the HFE p.C282Y mutation.5 Iron overload of explant livers has been associated in the former study with markedly reduced post-transplant survival due to fatal bacterial and fungal infections. Chronic hemolysis attributable to SCA and, consecutively, higher demands of red blood transfusions might contribute to secondary iron overload. Available histopathological data on explant hemosiderosis in SCA individuals indicate predominant hepatocellular and bile duct iron deposition (as opposed to the presented case with a mixed-type of iron overload), which points to a predominant role of mucosal iron hyperabsorption over transfusion-related hemosiderosis.6
As to the management of SCA, abstinence from alcohol and, if appropriate, listing for liver transplantation are key features, given the current lack of established medical treatment strategies.
Conflict of InterestNothing to declare.
Financial DisclosureAll authors disclose no financial relationships relevant to this publication.