Background and aims. Spontaneous bacterial peritonitis (SBP) often triggers acute-on-chronic liver failure (ACLF). Acute kidney injury (AKI) is frequent and correlates with higher mortality in such cases. The aim of this study is to evaluate the Acute Kidney Injury Network (AKIN) criteria in the prediction of death in cirrhotic patients after an episode of SBP.
Material and methods. Forty-six cirrhotic patients with SBP were included in a cohort study. Renal injury was estimated by AKIN criteria (grades 1, 2 or 3) to examine the association between AKI severity and mortality. Patients were followed-up for a mean of 13.22 months. Kaplan-Meier survival curve and the hazard ratio of mortality by Cox regression model were calculated accordingly to the AKIN criteria.
Results. The mean age of the included patients was 56.94 ± 9.49; 29 (63%) were male. Mean MELD score was 19.46 ± 6.16; 78.3% were Child-Pugh C. AKI occurred in 43.5% of patients (8.7, 17.4 and 17.4% respectively for AKIN criteria 1, 2 and 3). Inpatient mortality for AKIN 1, 2 and 3 was 50, 37.5 and 62.5 vs. 3.8% for patients without renal injury (p = 0.002, 0.001 and < 0.001 respectively). Patients with AKIN grades 1, 2 or 3 had no significant differences regarding MELD score (p = 0.893). The hazard ratio and 95% confidence interval of mortality for patients with AKI (AKIN grades 1, 2 and 3 grouped) were 3.41 (1.58-7.36).
Conclusions. AKIN criteria are useful to predict mortality in patients with SBP.
Spontaneous bacterial peritonitis (SBP) is a common and serious complication of patients with cirrhosis and ascites that occurs during the advanced stage of liver disease, with a prevalence of 10 to 30% in hospitalised patients.1–6 Its association with high rates of complications and mortality is precipitated by circulatory derangement causing liver failure and renal injury, which promotes in-hospital mortality of up to 30% despite resolution of the infection. Renal failure (RF) is the major predictor of mortality in SBP. Acute kidney injury (AKI) is common in patients with cirrhosis and ascites since renal dysfunction can occur as a result of systemic conditions that affect both the liver and the kidneys.7–15
The definition of AKI in cirrhosis is debated and there is no standardization. Levels of serum creatinine overestimate the renal function. The high cutoffs to the diagnosis of hepatorenal syndrome (HRS) underestimate the renal dysfunction and defers the treatment. The diagnosis of AKI in cirrhosis has been discussed by the Acute Kidney Injury Network (AKIN), which classifies renal dysfunction into grades of increasing severity based on changes in serum creatinine and/or urinary output.16–19 This score has been shown to predict clinical outcomes as mortality in cirrhotic patients with ascites.20,21
The MELD score is the foremost allocation model for liver transplant (LT) worldwide, but usually underestimates the severity of the liver disease in patients with portal hypertension and SBP,22–26 which suggests we need to improve prognostic tools in such cases. The present study was carried out to evaluate AKIN criteria in the prediction of death in patients with ascites and SBP.
Material and MethodsPatients aged 18 to 80 years with cirrhosis (clinical and/or biopsy proven) and SBP who were admitted to a single university hospital in southern Brazil (Hospital de Clínicas de Porto Alegre-HCPA) were evaluated. The inclusion criteria were:
- •
Ascitic fluid with ≥ 250 polymorphonuclear cells (PMNs)/mm3 and the absence of features suggestive of secondary bacterial peritonitis.
- •
The absence of other infections and gastrointestinal bleeding. Patients presenting with advanced neoplasms with expected survival of < 90 days and those HIV positive were excluded.
Patients were included in a cohort study and renal dysfunction was evaluated by the AKIN criteria into grades 1, 2 or 3 (Table 1). As the urinary output can be inconsistent, only the definition of change in serum creatinine was considered. Baseline creatinine was defined as the more recent stable outpatient measurement within 3 months prior to admission. Patients were then classified accordingly to the peak of AKIN stage during hospitalization. The evolution of AKI was classified in stable if there was no change at AKIN stage, progressive if there was increased in at least 1 AKIN stage, progressive with renal substitution therapy and regressive if there was decreased in at least 1 AKIN stage. MELD score was calculated at baseline and at the peak of creatinine level (peak of MELD score).
Classification of acute kidney injury according to AKIN.
| AKI stage | Serum creatinine criteria | Urinary output criteria |
|---|---|---|
| AKI stage 1 (risk) | Increase in serum creatinine of ≥ 0.3 mg/dL within 48 h or an increase of ≥ 150-200% (1.5-2-fold) from baseline | Urinary output < 0.5 mL/kg/h for > 6 h |
| AKI stage 2 (injury) | Increase in serum creatinine to 200-299% (2-3-fold) from baseline | Urinary output < 0.5 mL/kg/h for > 12 h |
| AKI stage 3 (failure) | Increase in serum creatinine to > 300% (> 3-fold) from baseline or serum creatinine of > 4 mg/dL with an acute increase of > 0.5 mg/dL or initiation of renal replacement therapy | Urinary output < 0.3 mL/kg/h for 24h or anuria for 12 h |
AKIN: Acute kidney injury network. AKI: Acute kidney injury.
Patients underwent a physical examination and laboratory (complete blood count, liver and renal tests, blood, urine and bedside ascitic fluid cultures, chest X-ray) before starting the treatment. The presence of additional infections was assessed based on the results of the initial tests. Routine blood tests to check renal function (urea, creatinine, sodium, potassium) were carried out daily. Plasma renin activity (PRA) by radioimmunoassay was measured at days 0 and 7 - blood samples were taken with the patient at rest and placed on ice. All patients were treated with intravenous cefotaxime for up to seven days. Diagnostic paracentesis was carried out 48 hours after the beginning of the treatment. A favourable response was a drop of > 50% in the number of the initial PMNs. The antibiotic protocol was modified if a cytological response was not obtained or according to the ascitic fluid culture.
Intravenous albumin (IV-A) was given to all patients on the first and third days of SBP treatment. During the first five days of treatment diuretics were forbidden. Large volume paracentesis above three litres was not performed during the treatment to prevent haemodynamic dysfunction and renal impairment. After the episode of SBP, patients received prophylactic antibiotics (norfloxacin or trimethoprim-sulfamethoxazole) and the usual treatment for ascites (2 g dietary sodium restriction and diuretics for grade 2 ascites or paracentesis with IV albumin replacement for grade 3 ascites). Patients with type 1 HRS were treated with IV terlipressin and albumin for up to fourteen days. After discharge, the patients were followed-up by a mean of 13.22 months.
Statistical analysisThe results are presented as means ± SD for the continuous variables with symmetric distribution or median and range (interquartile interval, percentile 25 and 75) for the continuous variables with asymmetric distribution and as counts and percentages for the categorical variables. Group comparisons were made with the chi-squared test and Fisher’s exact test for categorical variables and Student’s t-test and the Wilcoxon-Mann-Whitney test for continuous variables. Survival function was calculated by Kaplan-Meier according to AKIN criteria. Cox regression model was performed to estimate the hazard ratio of death regarding to AKIN stage, adjusted by Child-Pugh and MELD scores. A p value < 0.05 was considered significant. Analyses were performed with the PASW statistical package (SPSS version 18.0, SPSS, Chicago, IL). STROBE Check-list for cohort study was performed.
ResultsA total of 46 patients with cirrhosis, ascites and SBP were included into the study between March 2006 and May 2011. Baseline demographic, clinical and laboratory data are shown in table 2. Their mean age was 56.94 ± 9.49 years and there was a predominance of male gender (63%). Most of patients had HCV-related cirrhosis (67.4%) and evidences of severe liver dysfunction as shown by high rate of Child-Pugh C (78.3%) and mean MELD score (19.46). Cytological response occurred in 87.5% of patients. The other patients needed to expand the antibiotic regimem. There was no diagnosis of secondary bacterial peritonitis. Blood and ascites cultures were collected of 33 and 46 patients (71 and 100%) and were positive in 15.2 and 32.6% of them, respectively. Gram negative bacilli (Escherichia coli and Klebsiella pneumoniae) were the most frequent bacteria isolates from blood and ascites fluid (57.14 and 53.33%, respectively). Inpatient and 90-day mortality were 23.9% and 37%, respectively.
Baseline demographic, clinical and laboratory data of the 46 included patients.
| Total | |
|---|---|
| Age, years | 56.94 ± 9.49 |
| Gender, male | 29 (63) |
| MELD score | 19.46 ± 6.16 |
| Peak of MELD score | 21.65 ± 7.49 |
| Child-Pugh score | 10.78 ± 1.87 |
| Child-Pugh class C | 36 (78.3) |
| Mean arterial pressure, mmHg | 78.07 ± 11.94 |
| Hepatitis C cirrhosis | 31 (67.4) |
| Serum creatinine, mg/dL | 1.26 ± 0.55 |
| Serum sodium, mmol/L | 133.24 ± 5.70 |
| Serum bilirubin, mg/dL* | 3.45 (2.30-6.45) |
| INR | 1.71 ± 0.47 |
| Serum albumin, mg/dL | 2.60 ± 0.52 |
| Plasma renin activity, day 0, ng/mL/h* | 10.50 (3.82-20.52) |
| Plasma renin activity, day 7, ng/mL/h* | 5.96 (0.26-14.16) |
| Ascitic-fluid PMN, ×10-3/mm3 | 2962 ± 3114 |
| Acute kidney injury | 20 (43.5) |
| AKIN grade 1 | 4 (8.7) |
| AKIN grade 2 | 8 (17.4) |
| AKIN grade 3 | 8 (17.4) |
Data are presented as n (%), mean ± standard deviation, or median (range).
AKI occurred in 20 patients (43.5%), of whom 4 (8.7%), 8 (17.4%) and 8 (17.4%) had AKIN grade 1, 2 and 3 respectively. Type 1 HRS occurred in 6 patients (13%). AKI was stable in 4 patients (8.7%), progressive in 4 patients (8.7%), progressive with renal substitution therapy in 2 patients (4.3%) and regressive in 7 patients (21.7%).
Inpatient mortality was 3.8% for patients without AKI in comparison with 50%, 37.5% and 62.5% for patients with AKIN stage 1, 2 or 3 (p = 0.002, 0.001 and < 0.001, respectively). Inpatient death occurred in 10 out of 20 patients with AKI (50%), in comparison to 1 of 26 patients without AKI (3.8%) (p < 0.001).
90-day mortality was 20% for patients without AKI in comparison with 75%, 50% and 71.4% for patients with AKIN stage 1, 2 or 3 (p = 0.023, 0.021 and 0.008, respectively). Mortality at 90 days occurred in 12 of 19 patients with AKI (63.2%) in comparison with 5 of 25 patients without AKI (20%) (p = 0.009).
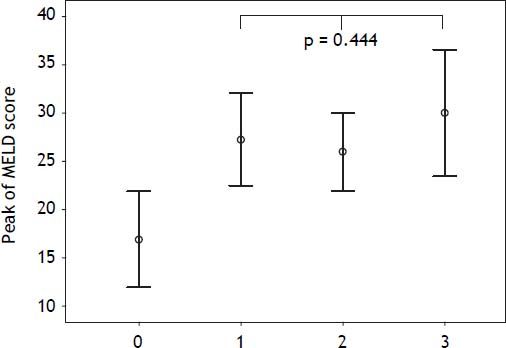
Mean MELD score was 16.69 ± 5.04 for patients without AKI and 24.5 ± 4.20, 22.50 ± 5.58 and 22.88 ± 6.89 for patients with AKIN stage 1, 2 or 3, respectively. There were no significant differences on MELD score among patients with AKIN stages 1, 2 or 3 (p = 0.893). Peak of MELD score was 16.88 ± 4.96 for patients without AKI and 27.25 ± 4.78, 26 ± 4.07 and 30 ± 6.56 for patients with AKIN stage 1, 2 or 3, respectively. Considering AKIN grades 1, 2 or 3, there were no significant differences regarding peak of MELD score (p = 0.444) (Figure 1).
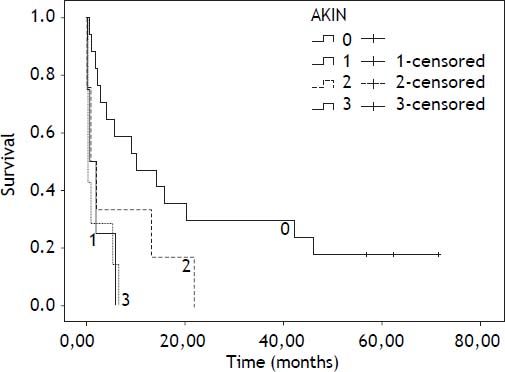
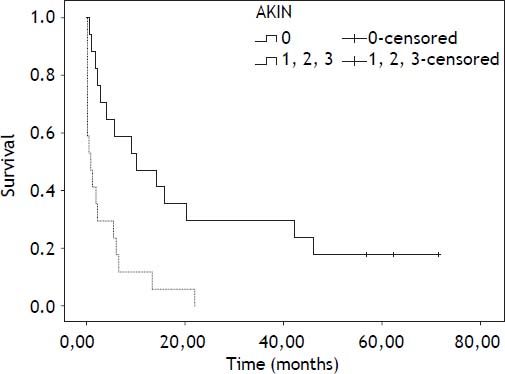
Mean follow-up was 13.22 months (6.12-20.32; 95% confidence interval). During this period 35 patients died (76.11%), 8 patients underwent LT (17.4%) and 3 remained alive (6.5%). Survival by Kaplan-Meier curves according AKIN are presented at figures 2 and 3.
The hazard ratio and 95% confidence interval of mortality for patients with AKI (AKIN grades 1, 2 and 3 grouped) were 3.41 (1.58-7.36) (Table 3). The hazard ratio of mortality for AKI corrected by MELD score is shown at table 4. The hazard ratio and 95% confidence interval of mortality for patients with AKIN stage 1, 2 or 3 were 4.13 (1.22-13.91), 2.5 (0.93-6.70) and 4.99 (1.8-13.08), respectively. Regarding AKI evolution, mortality was 50% for stable AKI, 100% for progressive or progressive requiring renal substitution therapy and 20% for regressive AKI.
SBP is the most frequent and life-threatening infection in patients with cirrhosis.4,6,11 AKI is common in these patients and is correlated with worse prognosis.5,12,16–19 Furthermore, infections are the most important and possibly underrecognized precipitant factors of acute-on-chronic liver failure (ACLF). Kidney function is almost universally altered in patients with ACLF due the underlying circulatory abnormalities.27,28 LT provides the only curative therapeutic option with favourable longterm results in patients with decompensated cirrhosis, especially after SBP. The MELD score is useful in predicting 3-month mortality in patients with cirrhosis. However, it has been criticized for several different reasons and may underestimate the severity in patients with ascites, SBP, and/or hepatic encephalopathy, among others. Beyond of MELD score limitations, the increase of LT waiting list mortality and the shortage of donor organs require efforts to improve allocation criteria.22–26
Isolated serum creatinine is notoriously inaccurate in the diagnosis of renal dysfunction in cirrhosis since patients with decompensated disease often present low serum creatinine levels relative to their glomerular filtration rate, owing to the reduced production of creatinine from creatine in the liver and the significant muscle wasting. Thus, serum creatinine can still be within the normal range despite significant renal dysfunction. Furthermore, the cut-off of serum creatinine usually applied to define acute RF or type 1 HRS (1.5 and 2.5 mg/dL, respectively) are very high, perhaps delaying appropriate diagnosis and management. Considering the limitations of isolated serum creatinine, in 2004 was developed the RIFLE criteria (R: risk, I: injury, F: failure, L: lesion, E: end-stage renal disease) for AKI which stratified acute renal dysfunction into grades of increasing severity based on changes in serum creatinine and/or urinary output.29 This criteria can predict clinical outcomes; the mortality increases along with its worsening. The definition of AKI was broadened by the Acute Kidney Injury Network, an independent collaborative network, to include an absolute increase in serum creatinine of 0.3 mg/dL within 48 h, since smaller increases in serum creatinine than those considered in the RIFLE classification have been shown to be associated with an adverse outcome,19 especially in cirrhotic patients admitted to ICU with multiorgan failure. There are new concepts and prognostic markers for renal dysfunction in cirrhosis and ACLF. This is particularly relevant for those awaiting liver transplantation who should be given a high priority at the liver transplant list.26–31
Carvalho, et al. have applied the AKIN criteria to predict in-hospital mortality in 198 cirrhotic patients with ascites. Overall mortality was 40.4%. AKI occurred in 46% of patients (41.9% AKIN 1, 2.5% AKIN 2 and 1.5% AKIN 3). We found similar rates of AKI, but our results were different from them, as most of our patients reached AKIN grades 2 and 3. Perhaps, this difference is related to the patients‘ profile, as they included a smaller number of patients with advanced cirrhosis (Child-Pugh C 45%). Both studies reported increased mortality with AKIN progression. Also, instead of taking as baseline the last outpatient creatinine, they have estimated AKIN criteria with results from the first 48 h of admission, which seems not to be adequate. Furthermore, in Carvalho’s study, AKIN was applied considering a bidirectional variation in creatinine and not only its increase.20
Belcher, et al. also evaluated AKIN prognostic value in cirrhotic patients regarding complications and mortality during hospitalization. They included, like us, patients with advanced cirrhosis (Child-Pugh C 65%). AKIN progression was related to mortality and medical and hepatic complications.21
In this study, we evaluated AKIN criteria in predicting mortality after SBP. Most of patients had high-risk SBP (creatinine > 1 g/dL or bilirubin > 4 mg/ dL). To the best of our knowledge, there are no similar studies to enable a comparison with our results.
Indeed, we found that mortality was significantly higher in patients with AKI, notably at AKIN stage 3, and there was no difference in MELD score in patients presenting with any stages of renal injury. It is remarkable that the hazard ratio of death has increased in patients with renal injury from AKIN 1 to 3 while at the same time MELD score was similar among the groups. Despite of creatinine being in the MELD score, there was no significant differences of MELD score peak among patients with AKIN stages 1, 2 and 3. The hazard ratio of mortality for patients with AKIN grade 3 was significant even after MELD score was corrected. The long-term survival after SBP was very low, mainly in patients with AKIN 3. These mortality rates were much higher than those predicted by MELD score. Therefore, MELD score seems not to be enough reliable on predicting mortality in cirrhotic patients after SBP. Those with severe AKI had higher risk of death and might need other criteria than MELD to assure a more fair liver allocation.
This study has some strength that should be emphasized: we have applied a modern classification of renal dysfunction and also stratified patients of higher mortality risk. The results have shown a large increase of mortality in patients with AKIN peak 3, and made clear that there MELD lacks ability to identify those with worse prognosis. On the other hand, there are limitations. The sample size is small and a type II error can be occurred. This can explain the results of mortality in AKIN group 2 in comparison with groups 1 and 3. Furthermore, AKIN criteria were performed without urinary output measurement and the cohort was retrospective and conducted at a single center.
ConclusionAKIN criteria are useful to predict mortality in patients with SBP. The role of AKIN in adjusting the albumin therapy and also for liver allocation in LT candidates after SBP needs to be addressed in further studies.
Declaration of InterestThere was no conflict of interest.
Financial DisclosureThe authors declare that they have nothing to disclose.