By using molecular surveillance of hepatitis A virus, we characterized for the first time a subgenotype IB imported case in Argentina, a country with universal vaccination since 2005. The case was a crew member of a cruise ship. We consider this a case alert because of its multiple implications.
Hepatitis A is one of the most common vaccine-preventable infections acquired during travel.1 In 2005, public health authorities in Argentina began a universal immunization program in 12-month-old children. With a vaccination coverage of 95% in 2006, the incidence of symptomatic viral hepatitis A in 2007 dropped sharply (> 80%).2 Subgenotype IA, the most prevalent in Latin America and in the world, is the only subgenotype circulating in Argentina and all the variants grouped in two large clades.3,4 Molecular epidemiology of hepatitis A virus (HAV) has proved valuable in identifying risk groups and tracing routes of transmission.4,5
Case ReportAs the National Reference Laboratory in Viral Hepatitis, we receive samples from different nets that contribute to the National Surveillance System. HAV RNA was detected with pairs of primers directed to different genomic regions, and positive products were sequenced and analyzed with phylogenetic programs, as previously described.3
An imported subgenotype IB was detected in a sample submitted from Ushuaia, a city in Argentina well-known as the southernmost city in the world. The case was a 28-year-old Polish male, member of the crew of a cruise-ship that left Miami, USA, on December 5, 2010, and traveled south along the South American Pacific and then along the Atlantic coast. This man got off the ship with acute hepatitis symptoms on January 10, 2011.
He reported being vaccinated with one dose against HAV in his country the year before, but this was unproven. Another crew member had got off in a Chilean port two weeks before with acute hepatitis symptoms. The only risk factor after an exhaustive epidemiological questionnaire - made first by the physician on board and then by the physician on land- was shellfish consumption on land in Mexico. This was the only time the patient had got off the ship since he had been hired two months before.
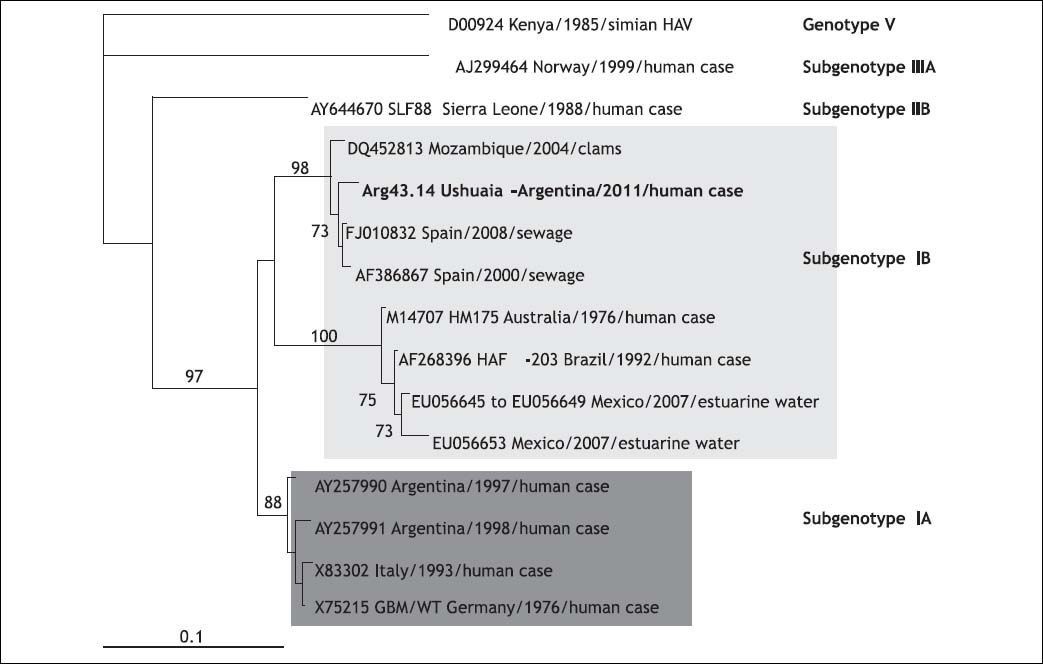
The sequences of a 456-bp-long fragment in the 5’NCR and a 350-bp-long fragment in VP1/2A were obtained (GenBank accession numbers: JX912999 and JX912998). The highest homology (98%) for the VP1/2A sequence was with two Spanish strains from sewage and with one from clams from Mozambique.6,7 Shellfish are able to ingest and concentrate HAV, and as a result become a reservoir for the spread of the virus.
Subgenotype IB has been found in Brazil and in imported Peruvian oysters in Europe. This subgenotype has been recovered from water samples in Mexico. A common 182-bp-long fragment from esturine sequences in the VP1/2A region showed 87% of homology with our imported case.8 The phylogenetic tree clearly demonstrated that they are not related and grouped in different clades (Figure 1).
Phylogenetic tree constructed by means of the neighbor-joining method, based on a 182-nucleotide fragment of the HAV VP1/2A junction region. Each reference viral strain is identified by its GenBank accession number, species and country of origin, and respective genotype and subtype. Numbers at the nodes indicate bootstrap percentages over 1,000 replicates (only values > 70% are shown). The bar indicates genetic distance.
An important epidemiological shift was observed in Argentina, previously considered a risky destination for travelers from low endemicity areas for HAV.1,9
The original incidence of reported HAV in Argentina fluctuated between 70.5-173.8 cases/100,000 between 1995-2004 respectively. In 2007, incidence dropped sharply to ~10 cases/100,000 in all age groups, confirming the experience gained in universal immunization programs elsewhere that indicated that effective immunization of toddlers will lead to widespread herd immunity.2
However, this case highlights that for those who remain susceptible, new transmission patterns (contaminated food and water, international travelers, injection drug users or men who have sex with men) could emerge and new variants of subgenotype IA or other subgenotypes could be introduced.3 It also reinforces that molecular surveillance is an important tool both for monitoring changes in patterns of disease transmission and for evaluating the impact of our program vaccination with a single dose.3 On the other hand, this case remarks the need that all susceptible people traveling for any purpose, frequency, or duration to countries with high or intermediate endemicity or to any destination should consider being vaccinated or receiving immunoglobulin (IG) before departure.1
Although Hepatitis A outbreaks on cruise ships have not been published, this case, which is related to an additional clinically compatible case, calls for an alert. The cruise ship market is the fastest-growing sector of the travel industry. In 2008, the North American cruise industry, which makes up most of the global cruise market, comprised 161 ships carrying more than 13 million passengers to destinations worldwide. Florida is the center of USA cruise ship travel, accounting for more than half of all USA departures. A typical cruise ship carries approximately 2,000 passengers and 800 crew members; however, cruise ship capacities continue to increase and can exceed 5,000 passengers and 2,000 crew members. Communicable diseases can be either introduced onboard by embarking travelers or acquired during visits to seaports with varying risks of exposure to infectious diseases. The crowded, semi-enclosed environment of the cruise ship facilitates transmission of communicable diseases, either person to person or from contaminated food, water, or environmental surfaces. Additionally, crew members, who are often from developing countries that may lack routine vaccination programs, can be sources of infection of vaccine-preventable diseases as hepatitis A, which has an incubation period that ranges from 15 to 50 days. So, this case also highlights that crew members should have documented proof of immunity to vaccine-preventable diseases.1,10
AcknowledgmentsThe authors want to thank to Mario Molinari, M.D. and Marta Acuña, BSc.
Financial SupportNo grants or other financial support.