Our aims were to validate the Brazilian Portuguese version of the Liver Disease Quality of Life Questionnaire instrument (LDQOL1.0) and evaluate this tool in non-cirrhotic patients. Methods: The LDQOL1.0 consists of the SF-36 generic measure of health-related quality of life (HRQOL) and 12 disease-specific dimensions for patients with liver disease. The Brazilian Portuguese version instrument was administered to 103 patients with liver disease. Reliability was analyzed by studying the internal consistency of the individual disease-specific dimensions of the LDQOL, as well as by studying the test-retest reliability in a sub-group of 74 patients who completed the questionnaire on two occasions 1 to 2 weeks apart. Validity was analyzed by determining the instrument’s ability to discriminate between groups of patients according to severity of the liver disease. Results: Internal consistency in the disease-specific dimensions was good (CACs 0.66-0.94), as well as test-retest reliability in all dimensions (ICCs of 0.64-0.96) p < 0.05. We found statistically significant differences in six domains when compensated cirrhotic patients, Child A, were compared with non-cirrhotic patients. We also found statistically significant differences when we compared cirrhotic patients evaluated assessed by Child-Pugh and Meld classifications. Patients with Child C and Meld ≥ 15 showed worse quality of life. Moderate ceiling and floor effects were found in some disease-specific dimensions. In Conclusions: the Brazilian Portuguese version of the LDQOL 1.0 is very useful for measuring HRQOL in liver diseases.
Abbreviations used:
LDQOL (Liver Disease Quality of Life Questionnaire instrument)
HRQOL (Health-related Quality of Life)
CAC (Cronbach’s Alpha Coefficients)
ICC (Intraclass Correlation Coefficient)
QOL (Quality of Life)
HCV (Hepatitis C Virus)
CHC (Chronic Hepatitis C)
MELD (Model for End-Stage Liver Disease)
IntroductionThe need to evaluate quality of life (QOL) originated in the social sciences with the advent of welfare. The shift from a social to an individual health problem arises from the spread of chronic diseases and the need to integrate measures that evaluate individual and community outcomes. 1
At present, the patient is considered ultimately responsible for major decisions about his/her own health. In addition, a patient’s subjective perception of his/her QOL is, in most cases, different from that of a professional.
The clinical paradigm, with its biomedical model, stresses the notion of QOL as a functional capacity focused on traditional variables such as physiological and clinical outcomes, and fails to consider other aspects of life such as carrying out daily activities or having good social relationships.2
Assessing health-related quality of life (HRQOL) is particularly meaningful in chronically ill subjects, especially those with a low probability of cure and who have to endure this condition for a lifetime.3
Standardized measures of HRQOL are increasingly used as a means of measuring the impact of disease and the effectiveness of treatments. Such measures may be either disease specific or generic. The Liver Disease Quality of Life Questionnaire instrument (LDQOL 1.0) is an questionnaire instrument developed to measure HRQOL in patients with different types of liver disease or patients submitted to liver transplant.4 Chronic liver disease ranges from asymptomatic chronic hepatitis to liver failure and transplantation. It is therefore important to understand the different levels and quality of experience in patients with differing severities of liver disease. Among these diseases, infection with the hepatitis C virus (HCV) has emerged as a public health problem. In addition, emerging literature suggests that, even in the absence of clinically significant liver disease, chronic HCV infection causes a consistent and clear impairment in HRQOL compared with that of healthy individuals.5,6 However, specific instruments to evaluate HRQOL in cases of liver disease are rarely used in medical practice. The instrument referred to here was recently translated and adapted into Brazilian Portuguese.7 The domains of the LDQOL are related to daily activities that HCV patients have to undertake. The objectives of this study were to validate the Brazilian Portuguese version of the LDQOL, and also to evaluate this tool in non-cirrhotic patients, especially those with chronic hepatitis C (CHC).
Patients and methodsInstrumentsThe LDQOL1.0 consists of a generic instrument (SF-36) and 12 disease-specific scales containing 75 items. The SF-36 was previously translated and validated for use in Brazilian Portuguese,8 and the Brazilian version of the questionnaire has been included here (appendix). The 12 disease-specific scales in LDQOL are: Symptoms of liver disease (17 items), Effects of liver disease (10 items), Concentration (7 items), Memory (6 items), Quality of Social Interaction (5 items), Health Distress (4 items), Sleep Problems (5 items), Loneliness (5 items), Hopelessness (4 items), Stigma of Liver Disease (6 items), Sexual Functioning (3 items), and Sexual Problems (3 items). The Brazilian Portuguese version of the disease specific questions questionnaire was drawn up using the standard recommended methodology of forward and back translation followed by testing in interviews with patients.7,9 The authors supplied the original version and authorized the translation.4 The punctuation of the questionnaire varies of 0-100 and higher scores means better quality of life.9
Study population. The Brazilian Portuguese version of the LDQOL was administered in the cross-sectional study to the sample of 103 patients with liver disease. The questionnaires were self-administered whenever possible. Other dates collected included age, gender, type of liver disease, Child-Pugh and MELD classification.
The reliability was evaluated applying the questionnaire in a sub-group of 74 on two occasions separated by approximately 1-2 weeks. The patients were clinically stable. Reliability was analyzed by studying the internal consistency of the individual disease-specific dimensions of the LDQOL, as well as by studying the test-retest reliability.
Preliminary validity testing was performed by analyzing the questionnaire’s ability to discriminate between groups of patients expected to differ in severity of liver disease. One hundred-three patients were analyzed, of whom 40 were non-cirrhotic with chronic hepatitis C (CHC) and 63 were cirrhotic with several etiologies, alcoholic liver disease, viral hepatitis, NASH (nonalcoholic steatohepatitis) and cryptogenic cirrhosis. The cirrhotic group were classified assessed by Child - Pugh and MELD classification, and 34 were Child A, 14 Child B and 15 Child C. In the non-cirrhotic group, all patients were submitted to liver biopsy and had moderate or severe CHC with indication for antiviral treatment. In accordance with the guidelines of the Brazilian Society of Pathology and Hepatology, all biopsy samples were semiquantified for the following parameters: architectural staging (0-4); grading of portal inflammation (0-4); interface hepatitis (0-4) and parenchymal necro-inflammatory lesions (0-4).12 The CHC patients with treatment indication presented architectural staging ≥ 2 and/or interface hepatitis > 2.12 The following comparisons were made:
- 1.
Non-cirrhotic vs Child A
- 2.
Child A vs Child B vs Child C
- 3.
MELD < 15 vs MELD ≥ 15
The internal consistency of the disease-specific dimensions was assessed using Cronbach’s alpha, and test-retest reliability was assessed using the intraclass correlation coefficient (ICC).13 The Kruskal-Wallis test was used for comparison among the three cirrhotic groups based on the severity of liver disease. The non-cirrhotic group and the compensated cirrhotic patients (Child A) were compared using the Mann-Whitney U-test. The quantitative parameters were assessed using the Student’s t-test. Statistical significance was inferred at the level of 5%. Ceiling and floor effects (the percentage of patients with maximum and minimum scores, respectively) were also calculated for each dimension.
ResultsPatient socio-demographic and clinical characteristics are shown in Table I. The internal consistency of disease-specific dimensions of the LDQOL was very good, with all coefficients being over 0.66 (Table II). Test-retest reliability was also good in all dimensions and all ICCs were statistically significant, p < 0.05 (Table III). The comparison by MELD classification showed statistical significance among patients with MELD scores < 15 and ≥ 15, p < 0.001. Worse quality of life was found in patients with Meld scores ≥ 15 for the following domains, symptoms of liver disease, effects of liver disease, sleep problems and stigma of liver disease (Figure 1). We also observed statistically significant differences in some domains when cirrhotic patients were compared by Child-Pugh classification. Worse quality of life was found in Child C patients (Figure 2). The dimension “sexual problems” was only evaluated in the groups Child A and Child B due low numbers of answer in the group Child C, only two. We didn’t found statistically significant differences among the two groups in relation to the scale “sexual problems” (p = 0.074). In spite of it the p value was marginally significant indicating a tendency to difference among these groups. In the other scales of the specific questions of the LDQOL statistically significant differences was found among the groups Child A, B and C (p < 0.05 in all of the comparisons), for following domains.
Sociodemographic and clinical characteristics of the patients.
| Patients (n) | (103) |
| Non-cirrhotics | (40) |
| Cirrhotics | (63) |
| Mean age, in years (SD)* | |
| Non-cirrhotics | 37 (10.8) |
| Cirrhotics | 46 (9.2) |
| Gender (% males) | |
| Non-cirrhotics | 12 (60) |
| Cirrhotics | 47 (64.4) |
| Child-Pugh (classification%) | |
| Child-A | 34 (53.98) |
| Child-B | 1z4 (22.22) |
| Child-C | 15 (23.80) |
| Etiology of cirrhosis (%) | |
| Viral hepatitis | 21 (33.33) |
| Alcohol | 30 (47.62) |
| 7 (11.11) | |
| Nash (nonalcoholic steatohepatitis) | |
| Cryptogenic | 5(7.94) |
Internal Consistency of Disease-Specific Dimensions in the Brazilian Portuguese Version of the LDQOL1.0 Questionaire instrument
| N = 34 compensated cirrhotic patients and 40 non-cirrhotic patients Dimension | |||
|---|---|---|---|
| Dimension | Cronbach’s | % Scoring the floor | % Scoring the ceiling |
| Symptoms related to liver disease | 0.76 | 0.0 | 10.4 |
| Effects of liver disease on activities of daily living | 0.84 | 0.0 | 9.6 |
| Concentration | 0.84 | 0.0 | 10.0 |
| Memory | 0.94 | 0.0 | 10.0 |
| Quality of social interaction | 0.66 | 1.4 | 12.3 |
| Health Distress | 0.76 | 10.2 | 3.2 |
| Sleep problems | 0.74 | 3.5 | 0.0 |
| Loneliness | 0.84 | 1.4 | 19.8 |
| Hopelessness | 0.82 | 2.8 | 12.7 |
| Stigma of liver disease | 0.72 | 1.2 | 20.4 |
| Sexual function | 0.76 | 24.7 | 20.2 |
| Sexual problems | 0.74 | 17.8 | 24.3 |
Test-retest reliability of disease-specific dimensions in the Brazilian Portuguese Version of the LDQOL1.0 Questionnaire instrument. N = 34 compensated cirrhotic patients and 40 non-cirrhotic patients.
| Dimension | ICC |
|---|---|
| Symptoms related to liver disease | 0.79* |
| Effects of liver disease on activities of daily living | 0.95* |
| Concentration | 0.94* |
| Memory | 0.96* |
| Quality of social interaction | 0.86* |
| Health distress | 0.91* |
| Sleep problems | 0.64* |
| Loneliness | 0.68* |
| Hopelessness | 0.85* |
| Stigma of liver disease | 0.69* |
| Sexual function | 0.83* |
| Sexual problems | 0.77* |
ICC; intraclass correlation coefficient
Questionnaire LDQOL, 1.0. Comparison by MELD classification - cirrhotic patients. N = 63. According to the MELD classification, statistically significant differences were found in the following domains of the LDQOL: symptoms of liver disease, effects of the liver disease (p = 0.015), Sleep Problems (p = 0.009) and Stigma of liver disease (p = 0.002). The scores of the patients with MELD ≥ 15 were significantly lower than those with MELD < 15.
In the dimensions “symptoms of liver disease” (p < 0.001), “concentration” (p < 0.001) and “memory” (p < 0.001), the patients of the group Child A presented their scores significantly higher than the patients of the other groups, Child B and Child C, that didn’t differ in a significant way; in the dimensions “effects of liver disease” (p < 0.001), “sleep” (p < 0.001) and “sexual function” (p < 0.001), the patients of the group Child C presented scores significantly lower than the patients of the other groups, Child A and Child B, that didn’t differ in a significant way; in the dimensions “quality of the social interaction” (p = 0.007), “loneliness” (p = 0.008) and “hopelessness” (p = 0.025), the patients of the group Child A presented higher scores than the patients of the group Child C and the group Child B didn’t differ in significant way of the other groups; in the dimensions “Health distress” (p < 0.001) and “stigma of liver disease” (p < 0.001), the patients of the group Child A presented higher scores than the patients of the group Child B, that presented higher scores than the patients of the group Child C (Figure 2).
Non-cirrhotic and compensated cirrhotic patients (Child A) showed statistically significant HRQOL differences in six domains: symptoms of liver disease, memory, concentration, health distress, sleep and stigma of liver disease. The scores of the compensated cirrhotic patients in five of these domains were lower than those of non-cirrhotic patients, p < 0.05 (Figure 3). Just in one domain, which is stigma of liver disease, the group of non-cirrhotic patients constituted by HCV carriers, demonstrated lower punctuations. We found some ceiling and floor effects in some of the disease-specific dimensions (Table II). In 3.8% of the subjects, LDQOL disease target scores were missing, what is considered acceptable for missing data.
DiscussionThe aims of this study were: 1) To validate the Brazilian Portuguese version of the Liver Disease Quality of Life Questionnaire instrument. 2) Give an insight into the differences in disease-specific HRQOL instruments using LDQOL among non-cirrhotic and cirrhotic patients. Chronic liver disease leads to significant morbidity and mortality; its manifestations affect not only the physical symptoms but also the psychological and social functioning of affected individuals.14,15 Some authors have developed a disease-targeted HRQOL measure for persons with chronic liver disease, such as the “Chronic Liver Disease Questionnaire” produced in 1999.16 However, this measure may not capture potentially important areas. A disease-targeted HRQOL measure with 15 items was also developed in 1998, specifically for patients with chronic hepatitis C virus.17 As with the “Chronic Liver Disease Questionnaire” produced in 1999, this instrument also excludes some aspects of liver disease and problems relating to chronic HCV carriers. The LDQOL includes domains such as memory, concentration, sexual functioning, social functioning and selfperceived stigma of liver disease. These characteristics are very important because a disease-targeted measure should be able to demonstrate its value in providing additional HRQOL information. LDQOL 1.0 is able to provide unique information that a generic instrument alone would not capture in persons with chronic liver disease. This study was conducted in order to apply the instrument in patients with and without cirrhosis and to compare different levels of liver disease. We believe that this disease targeted instrument will be useful in studies that attempt to evaluate the HRQOL outcomes of patients before and after liver transplantation,18 as well as in evaluating several features of the different stages of liver diseases and the impact of their respective medical treatments, such as interferon in chronic hepatitis C. Nowadays, it is understood that HCV carriers show many physical and emotional alterations before and during treatment.19,20 We have demonstrated statistically significant differences between different stages of liver disease recorded by Child-Pugh and Meld classification as the original authors of this questionnaire,4 however, between cirrhotic and non-cirrhotic patients. is the first time. We have shown that the results of the disease-specific domains of the LDQOL worsened with worsening of the liver disease stage. This tool has proven to be appropriate in evaluating these issues.
Further testing of other types of validity, as well as the instrument’s sensitivity to change, is required.
This is the first validation study of the new Brazilian Portuguese version of the LDQOL 1.0. In this sample, the new language version showed very good internal consistency and test-retest reliability, and an ability to discriminate between patients classified according to the severity of the disease. including mild liver disease.
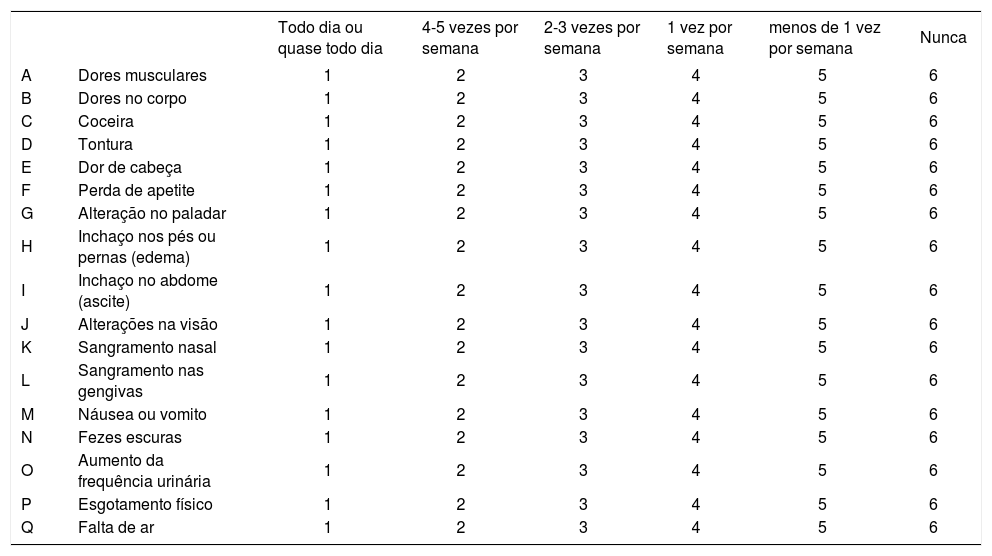
Appendix - Translated version-Questionário sobre qualidade de vida nas doenças hepáticas (LDQOL 1.0). Questionário sobre qualidade de vida nas doenças hepáticas (LDQOL 1.0)1. Estas questões são sobre sintomas ou problemas de saúde que você pode ter ou não. Nas últimas 4 semanas, quantas vezes você experimentou cada um dos seguintes sintomas? (sejam causados por sua doença hepática ou qualquer outro problema).
| Todo dia ou quase todo dia | 4-5 vezes por semana | 2-3 vezes por semana | 1 vez por semana | menos de 1 vez por semana | Nunca | ||
|---|---|---|---|---|---|---|---|
| A | Dores musculares | 1 | 2 | 3 | 4 | 5 | 6 |
| B | Dores no corpo | 1 | 2 | 3 | 4 | 5 | 6 |
| C | Coceira | 1 | 2 | 3 | 4 | 5 | 6 |
| D | Tontura | 1 | 2 | 3 | 4 | 5 | 6 |
| E | Dor de cabeça | 1 | 2 | 3 | 4 | 5 | 6 |
| F | Perda de apetite | 1 | 2 | 3 | 4 | 5 | 6 |
| G | Alteração no paladar | 1 | 2 | 3 | 4 | 5 | 6 |
| H | Inchaço nos pés ou pernas (edema) | 1 | 2 | 3 | 4 | 5 | 6 |
| I | Inchaço no abdome (ascite) | 1 | 2 | 3 | 4 | 5 | 6 |
| J | Alterações na visão | 1 | 2 | 3 | 4 | 5 | 6 |
| K | Sangramento nasal | 1 | 2 | 3 | 4 | 5 | 6 |
| L | Sangramento nas gengivas | 1 | 2 | 3 | 4 | 5 | 6 |
| M | Náusea ou vomito | 1 | 2 | 3 | 4 | 5 | 6 |
| N | Fezes escuras | 1 | 2 | 3 | 4 | 5 | 6 |
| O | Aumento da frequência urinária | 1 | 2 | 3 | 4 | 5 | 6 |
| P | Esgotamento físico | 1 | 2 | 3 | 4 | 5 | 6 |
| Q | Falta de ar | 1 | 2 | 3 | 4 | 5 | 6 |
2. Algumas pessoas se incomodam com os efeitos das doenjas hepáticas em sua vida diária, enquanto outras não se incomodam. Quanto cada um dos seguintes efeitos incomodou você, nas últimas 4 semanas, nas seguintes áreas:
| Intolerável | incomoda muito | moderadamente | um pouco | não incomoda | não se aplica | ||
|---|---|---|---|---|---|---|---|
| A | Restrição a líquidos | 1 | 2 | 3 | 4 | 5 | 6 |
| B | Restrijão alimentar | 1 | 2 | 3 | 4 | 5 | 6 |
| C | Habilidade de executartarefas domésticas | 1 | 2 | 3 | 4 | 5 | 6 |
| D | Ir a eventos sociais fora de casa | 1 | 2 | 3 | 4 | 5 | 6 |
| E | Executar alguma atividade de lazer ou recreação dentro de casa | 1 | 2 | 3 | 4 | 5 | 6 |
| F | Habilidade de viajar | 1 | 2 | 3 | 4 | 5 | 6 |
| G | Vida sexual | 1 | 2 | 3 | 4 | 5 | 6 |
| H | Medicamentos | 1 | 2 | 3 | 4 | 5 | 6 |
| O quanto você concorda com a seguinte afirmativa | |||||||
| Concordo muito | Concordo em parte | Não sei ao certo | Discordo em parte | Discordo totalmente | |||
| I | Muito do meu tempo é gasto lidando com minha doença hepática | 1 | 2 | 3 | 4 | 5 | |
| Quanto do seu tempo nas últimas 4 semanas… | |||||||
| J | A sua doença hepática fez com que perdesse o humor? | Todo o tempo 1 | A maior parte do tempo 2 | Algumas vezes 3 | Raríssimas vezes | Nunca 5 | |
As questões seguintes são a respeito de problemas de concentração que você possa ter.
Quanto do seu tempo, nas últimas 4 semanas, você encontrou dificuldades em…
| Todo o tempo | A maior parte do tempo | Algumas vezes | Raríssimas vezes | nunca | ||
|---|---|---|---|---|---|---|
| A | Concentrar-se na conversa | 1 | 2 | 3 | 4 | 5 |
| B | Concentrar-se na execução de alguma tarefa | 1 | 2 | 3 | 4 | 5 |
| C | Executar atividades envolvendo concentração e raciocínio | 1 | 2 | 3 | 4 | 5 |
4. Quanto do seu tempo, nas últimas 4 semanas, você…
| Todo o tempo | A maior parte do tempo | Algumas vezes | Raríssimas vezes | nunca | ||
|---|---|---|---|---|---|---|
| a | Teve dificuldade em manter a concentração numa atividade prolongada? | 1 | 2 | 3 | 4 | 5 |
| b | Ficou confuso? | 1 | 2 | 3 | 4 | 5 |
| c | Reagiu vagarosamente a alguma coisa dita ou feita? | 1 | 2 | 3 | 4 | 5 |
| d | Teve dificuldade em raciocinar ou resolver problemas? | 1 | 2 | 3 | 4 | 5 |
As seguintes questões são sobre memória:
Quanto do seu tempo, nas últimas 4 semanas, você experimentou dificuldades em se lembrar de…
| Todo o tempo | A maior parte do tempo | Algumas vezes | Raríssimas vezes | nunca | ||
|---|---|---|---|---|---|---|
| a | Nome de pessoas | 1 | 2 | 3 | 4 | 5 |
| b | Onde você pôs as coisas | 1 | 2 | 3 | 4 | 5 |
| c | Alguma coisa que alguém te falou/disse | 1 | 2 | 3 | 4 | 5 |
| d | Algo que você leu recentemente. Ex: o jornal pela manhã | 1 | 2 | 3 | 4 | 5 |
6. Quanto do seu tempo, nas últimas 4 semanas, você…
Questões sociais
Quanto do seu tempo, nas últimas 4 semanas, você…
| Todo o tempo | A maior parte do tempo | Algumas vezes | Raríssimas vezes | nunca | ||
|---|---|---|---|---|---|---|
| a | Isolou-se das pessoas? | 1 | 2 | 3 | 4 | 5 |
| b | Foi carinhoso com as pessoas? | 1 | 2 | 3 | 4 | 5 |
| c | Irritou-se com as pessoas? | 1 | 2 | 3 | 4 | 5 |
| d | Pediu coisas não razoáveis a seus amigos ou membros da família? | 1 | 2 | 3 | 4 | 5 |
| e | Foi uma pessoa muito comunicativa? | 1 | 2 | 3 | 4 | 5 |
Preocupação com a doença
Quanto do seu tempo, nas últimas 4 semanas, você…
| Todo o tempo | A maior parte do tempo | Algumas vezes | Raríssimas vezes | nunca | ||
|---|---|---|---|---|---|---|
| a | Sentiu-se desencorajado em virtude de sua doença hepática? | 1 | 2 | 3 | 4 | 5 |
| b | Sentiu-se frustrado em virtude de sua doença hepática? | 1 | 2 | 3 | 4 | 5 |
| c | Preocupou-se com sua doença hepática? 1 | 1 | 2 | 3 | 4 | 5 |
| d | Sentiu-se depreciado em virtude de sua doença hepática? | 1 | 2 | 3 | 4 | 5 |
O próximo conjunto de perguntas é sobre suas funções sexuais e seu grau de satisfação com elas.
10. Quanto à doença hepática interferiu nos eus relacionamentos sexuais:
11. Você manteve alguma relação sexual nas últimas 4 semanas?
12. Quão problemático foi para você cada um dos seguintes itens nas 4 últimas semanas: Homens: responder de (a) a (c) Mulheres: responder de (d) a (f)
| Sem problema | Pequena dificuldade | Com alguma dificuldade | Muita dificuldade | ||
| a | Dificuldade em conseguir ou manter uma ereção | 1 | 2 | 3 | 4 |
| b | Dificuldade em atingir orgasmo | 1 | 2 | 3 | 4 |
| c | Habilidade de satisfazer sexualmente a parceira | 1 | 2 | 3 | 4 |
| d | Lubrificação inadequada | 1 | 2 | 3 | 4 |
| e | Dificuldade em atingir orgasmo | 1 | 2 | 3 | 4 |
| f | Habilidade de satisfazer sexualmente o parceiro | 1 | 2 | 3 | 4 |
13. De um modo geral, qual seu grau de satisfação com suas funções sexuais nas últimas 4 semanas?
Sono
Por quanto tempo, nas últimas 4 semanas, você…
| Todo o tempo | A maior parte do tempo | Algumas vezes | Raríssimas vezes | nunca | ||
|---|---|---|---|---|---|---|
| a | Dormiu o suficiente para se sentir descansado pela manhã? | 1 | 2 | 3 | 4 | 5 |
| b | Sentiu sonolência durante o dia? 1 | 1 | 2 | 3 | 4 | 5 |
| c | Teve dificuldade de se manter acordado durante o dia? | 1 | 2 | 3 | 4 | 5 |
| d | Cochilou (5 minutos ou mais) durante o dia? | 1 | 2 | 3 | 4 | 5 |
| e | Dormiu a quantidade de tempo que necessita? | 1 | 2 | 3 | 4 | 5 |
Isolamento
Quanto do seu tempo, nas últimas 4 semanas, você…
| Todo o tempo | A maior parte do tempo | Algumas vezes | Raríssimas vezes | nunca | ||
| a | Não teve companhia | 1 | 2 | 3 | 4 | 5 |
| b | Não teve ninguém com quem contar | 1 | 2 | 3 | 4 | 5 |
| c | Sentiu-se abandonado | 1 | 2 | 3 | 4 | 5 |
| d | Sentiu-se isolado dos outros | 1 | 2 | 3 | 4 | 5 |
| e | Conseguiu encontrar companhia quando precisou | 1 | 1 | 3 | 4 | 5 |
Esperança
Quanto você concorda com as seguintes afirmativas:
| Concordo muito | Concordo em parte | Não sei ao certo | Discordo em parte | Discordo totalmente | ||
|---|---|---|---|---|---|---|
| a | Agora planejo menos o futuro do que antes da doença hepática | 1 | 2 | 3 | 4 | 5 |
| b | Tenho grande fé no futuro | 1 | 2 | 3 | 4 | 5 |
| c | Meu futuro parece sombrio | 1 | 2 | 3 | 4 | 5 |
| d | Encaro o futuro com esperança | 1 | 2 | 3 | 4 | 5 |
17. O quanto você concorda com as seguintes afirmativas:
| Concordo muito | Concordo em parte | Não sei ao certo | Discordo em parte | Discordo totalmente | ||
|---|---|---|---|---|---|---|
| a | Algumas pessoas me evitam por causa de minha doença | 1 | 2 | 3 | 4 | 5 |
| b | Sinto vergonha de minha aparência. | 1 | 2 | 3 | 4 | 5 |
| c | Evito me expor em virtude de minha doença hepática | 1 | 2 | 3 | 4 | 5 |
| d | Algumas pessoas sentem-se incomodadas quando estão comigo por causa de minha doença hepática | 1 | 2 | 3 | 4 | 5 |
| e | Minha doença faz com que eu me sinta deslocado em público | 1 | 2 | 3 | 4 | 5 |
| f | Sinto-me prejudicado e incompleto em virtude de minha doença hepática | 1 | 2 | 3 | 4 | 5 |