Purpose. Chronic hepatitis C virus (HCV) is a major problem affecting up to 170 million people worldwide. Two protease inhibitors have recently been approved that will revolutionize treatment. Our objective was to summarize and evaluate the literature pertaining to the pharmacokinetics of boceprevir and telaprevir, in order to provide clinicians with insight into the management of actual and potential drug interactions.
Summary. A standardized search using MEDLINE (1948-November 2011), EMBASE (1980-November 2011), IPA (1970-November 2011), Google, and Google Scholar that combined the search terms boceprevir, telaprevir, pharmacokinetics, drug interaction, and drug metabolism was performed. Manual reference searches of chosen articles were completed. Monographs and articles, conference proceedings, and abstracts were evaluated. Boceprevir and telaprevir are both substrates and inhibitors of cytochrome P450 3A4 and telaprevir is a substrate of p-glycoprotein. Levels of boceprevir are decreased in patients taking efavirenz but effects with other antiretrovirals are minimal or unknown. Coadministration with efavirenz may compromise telaprevir levels and should be avoided. Telaprevir may increase levels of cyclosporine, tacrolimus, atorvastatin, and amlodipine, which may expose patients to increased adverse effects.
Conclusions. Significant drug-drug interactions occur with both boceprevir and telaprevir. Until studies are reported and experience is gained with these agents, clinicians will need to be careful when administering in high-risk populations and those receiving chronic therapy with interacting agents. Studies are urgently needed in HIV patients taking antiretrovirals and patients taking chronic immunosuppresion as these populations are at increased risk of experiencing clinically significant interactions.
Chronic hepatitis C virus (HCV) is a major health problem affecting up to 170 million people world-wide.1 While many are not aware of infection, others become symptomatic and go on to develop liver cirrhosis which may lead to transplantation or premature death. These complications are associated with significant morbidity, as well as stress and psychological suffering. The only treatment available prior to 2011 was pegylated interferon and ribavirin.2 When administered together for 48 weeks, these agents were able to produce clinical cure in approximately 40-50% of patients with genotype-1 infection.3,4 For those who failed previous treatment, the likelihood of success with a second course, while reasonable for those who relapsed after previous response, is dismal at < 20% for those who were non-responders.5 The low rates of success in genotype 1 infection meant any patients had to manage complications of liver disease, with significant morbidity and mortality, or await transplantation. Of those with chronic HCV infections, two special groups, those with HIV co-infection and those who have undergone liver transplantation, where post-transplant HCV recurrence is universal, are well-recognized to have an accelerated natural history of the disease.6,7 Unfortunately, treatment with pegIFN and ribavirin in these groups is far less likely to be associated with treatment success compared to the non-HIV and non-transplant patient populations.8,9
In 2011, two new licensed agents have changed the management of hepatitis C infection. Boceprevir and telaprevir are both orally administered inhibitors of the HCV protease NS 3/4A. When combined with peginterferon and ribavirin for genotype-1 infection, success rates have increased to 68-75% for treatment naive and doubled to 60-65% of treatment experienced patients.10–13 Given the significant improvements in response rates for these difficult to treat patients, these protease inhibitors, in combination with peginterferon and ribavirin, are now the best treatment option for the treatment of HCV genotype 1 infection.
As with any new agent, the potential for drug-drug interactions needs to be assessed. Both of these agents are substrates and inhibitors of the cytochrome P450 3A (CYP3A) metabolic pathway.14,15 As many commonly used medications utilize the CYP3A metabolic pathway, the potential for many drug-drug interactions exists and the clinical significance of these interactions needs to be assessed to ensure optimal patient outcomes.
Two populations of particular interest are patients infected with the human immunodeficiency virus (HIV) and those requiring chronic immunusuppression, such as solid organ transplant patients. Many commonly used antiretrovirals for the treatment of HIV affect the CYP3A metabolic pathway, or are themselves substrates. The same is also true for immunosuppressive agents. These agents have narrow therapeutic windows in which optimal efficacy and safety can be achieved. The impact of introducing an agent that may interact with HIV or immunosuppression therapy, such as boce-previr or telaprevir, may be significant, resulting in treatment failure or development of serious adverse events. As substrates of CYP3A, boceprevir and telaprevir are also vulnerable to the influence of antiretrovirals and immunosuppressants on this pathway. It is therefore essential to characterize the clinical significance of any potential interaction, in order to achieve the best possible patient outcomes.
The objective of this review is to summarize and evaluate the literature pertaining to the pharmaco-kinetics of boceprevir and telaprevir in order to provide clinicians with insight into the management of actual and potential drug interactions. As of this writing, neither boceprevir nor telaprevir are licensed for use in patients co-infected with HIV or post-transplantation. Definitive clinical trials in these special populations are either ongoing or are in the planning stages. Given the severity of HCV infection in the post-transplant setting and in patients co-infected with HIV, it is anticipated that some patients from these special groups will be considered for off-label use of these antiviral agents.
Data sourcesA standardized search using MEDLINE (1948-Au-gust 2011), EMBASE (1980-August 2011), IPA (1970-August 2011), Google, and Google Scholar that combined the search terms boceprevir, telaprevir, pharmacokinetics, drug interaction, and drug metabolism was performed. Articles, conference proceedings, and abstracts that described pharmacokinetics and drug-drug interactions between boceprevir, telaprevir, and other agents were identified. Manual reference searches of chosen articles were completed to identify articles missed by the electronic search. Monographs produced by the manufacturer were also reviewed.
PharmacokineticsFew pharmacokinetic studies have been completed with boceprevir. According to manufacturer data, boceprevir is readily absorbed following oral administration with a median time to maximum serum concentration (Tmax) of 2 h. Food enhances absorption up to 60% at steady-state but no effects were seen when assessed for meal type (fat content) or timing in comparison to food intake (before, during, or after a meal). The mean apparent volume of distribution is 717 L and it is not highly protein bound (75% after a single oral dose). Boceprevir is primarily metabolized by the aldoketoreductase (AKR)-mediated pathway to inactive ketone metabolites. It is also a substrate of CYP3A4/5 and is an inhibitor of this enzyme. It has a mean plasma half-life of 3.4 h and therefore reaches steady state after approximately 1 day of three times a day dosing. There is minimal elimination of unchanged drug in the urine.14
Telaprevir is administered orally and is a substrate of the gastrointestinal efflux transporter p-glycoprotein. Food increases absorption and it is recommended to be taken with food to maximize this benefit. It is minimally bound to plasma proteins and has an approximate apparent volume of distribution of 252 L. It is extensively metabolized by hepatic hydrolysis, reduction and oxidation through the CYP3A enzyme pathway. It also acts as a potent inhibitor of the CYP 3A enzyme. It is primarily eliminated in the feces and has a mean plasma half-life of 8-11 h.15
Drug InteractionsBoceprevirBy utilizing multiple routes of metabolism (aldo-ketoreductase and CYP3A), boceprevir is less prone to drug interactions. A summary of selected interactions is reported in table 1. Manufacturer studies have shown coadministration with aldoketoreductase inhibitors (ibuprofen and diflunisal) does not result in clinically significant changes to boceprevir exposure.14 There is therefore no need to avoid coadministration.
Established and theoretical drug-drug interactions with boceprevir.
| Drug | Effect on Boceprevir | Effect on Drug | Comments |
|---|---|---|---|
| • HIV-Antiretrovirals | |||
| Efavirenz16 | ↓ | ↑ | Avoid combination. |
| Tenofovir16 | No effect | ↑ | No dosage adjustment necessary. |
| Ritonavir16 | ↓ | - | No dosage adjustment necessary. |
| Effects unknown in combination with other HIV protease inhibitors. | |||
| • Immunosuppressants | |||
| Cyclosporine | Unknown | ↑ | Expected increase in cyclosporine exposure. Therapeutic drug monitoring indicated for dose optimization. |
| Tacrolimus | Unknown | ↑ | Expected increase in tacrolimus exposure. Therapeutic drug monitoring indicated for dose optimization. |
| Sirolimus | Unknown | ↑ | Expected increase in sirolimus exposure. Therapeutic drug monitoring indicated for dose optimization. |
| • Other agents | |||
| Drospirenone16 | - | ↑ | Combination should be avoided. |
| Ethinyl estradiol16 | - | ↓ | Clinical significance unknown. Non-hormonal contraception should be used while taking boceprevir. |
Drug interaction studies have assessed pharmacokinetic changes between boceprevir and three antiretrovirals. When coadministered with the non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (a CYP3A inducer), Cmin levels of boceprevir decreased by 44%.16 It is unknown if this would result in HCV treatment failure so coadministration should be avoided until further evaluations are complete. Tenofovir, a nucleotide analog reverse transcriptase inhibitor (NRTI) does not appear to significantly alter the pharmacokinetics of boceprevir.16 Similarly, the HIV protease inhibitor (PI) ritonavir does not appear to interact with boceprevir.16 However, the effects of HIV protease inhibitors with or without ritonavir boosting are unknown and should be avoided until experience is gained with these agents.
There are currently no available drug interaction studies with immunosuppressant agents. Plasma concentrations of the calcineurin inhibitors cyclosporine and tacrolimus would be expected to increase due to inhibition of CYP3A by boceprevir. Cyclosporine is a substrate of CYP 3A but is also an inhibitor,17 and therefore may potentially increase the levels of boceprevir, resulting in increased frequency of adverse events including anemia. If coadministration cannot be avoided, careful therapeutic drug monitoring, and likely empiric dose reduction of these agents is indicated to ensure optimal efficacy and avoid dose related toxicities. While no data is available, it is our opinion that co-administration of boce-previr and the mammalian target of rapamycin inhibitor (mTOR) sirolimus should be avoided. The long half-life of sirolimus of 60 h, as well as the significant adverse effect of anemia would make concurrent therapy with the HCV protease inhibitors difficult to manage and may provide additive toxicity.18
Potential interactions exist between boceprevir and other agents that utilize and affect the CYP3A pathway. When coadministered with ketoconazole, a potent CYP3A inhibitor, boceprevir exposure was increased (131% increase in AUC, 41% increase in Cmax).16 The clinical effects of this interaction are unknown. Oral contraceptive agents also utilize the CYP3A pathway. Drospirenone levels increased when administered with boceprevir (AUC(0-24) and Cmax increased 99% and 57% respectively) and put patients at risk of adverse events.16 These agents should not be used concomitantly. Conversely, ethinyl estradiol concentrations were minimally affected (AUC decrease of 24%).16 Until more information is obtained, use of non-hormonal methods of birth control is preferred during treatment with boceprevir. Unfortunately, no data is available assessing theoretical interactions with potent inducers (eg. rifampin, phenytoin) or inhibitors (eg. clarithromycin, voriconazole) of the CYP system.
TelaprevirTelaprevir is both a substrate and potent inhibitor of CYP3A and is prone to many drug-drug interactions with other agents that utilize or affect this metabolic pathway. It may displace medication from plasma proteins, which may decrease plasma concentrations of concurrently administered medications which are highly protein bound.15 It is also a substrate of p-glycoprotein and may inhibit or saturate this transporter and higher concentrations of substrates may be observed. Few studies have assessed clinical effects of these interactions but recommendations can be made based on preliminary data. When initiating any potentially interacting agent, the most current data should be obtained to ensure appropriate dosage adjustments and monitoring occurs. Potential and actual drug interactions for commonly used agents are presented in table 2.
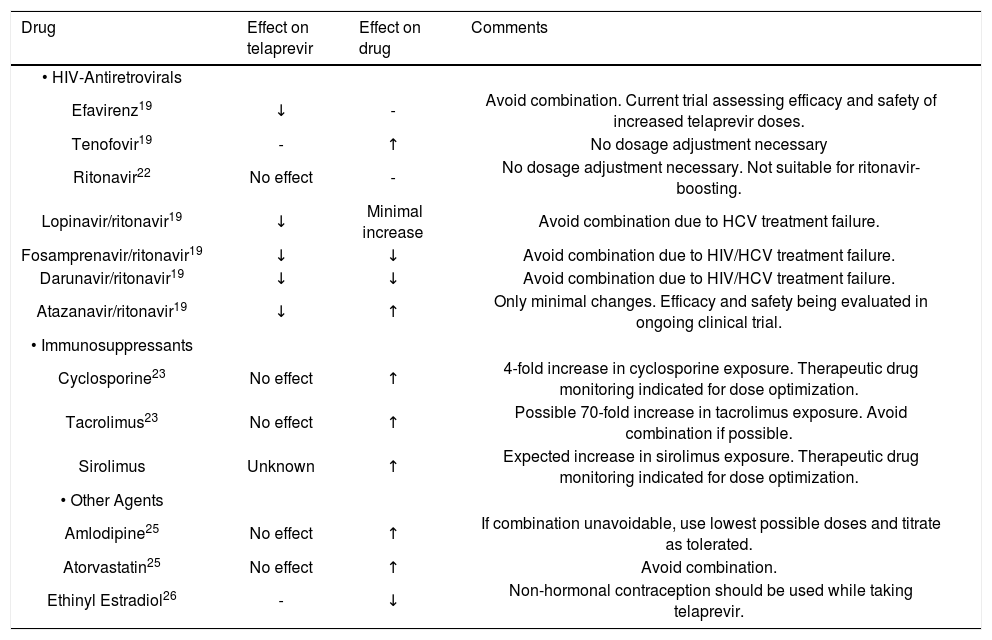
Established and theoretical drug-drug interactions with telaprevir.
| Drug | Effect on telaprevir | Effect on drug | Comments |
|---|---|---|---|
| • HIV-Antiretrovirals | |||
| Efavirenz19 | ↓ | - | Avoid combination. Current trial assessing efficacy and safety of increased telaprevir doses. |
| Tenofovir19 | - | ↑ | No dosage adjustment necessary |
| Ritonavir22 | No effect | - | No dosage adjustment necessary. Not suitable for ritonavir-boosting. |
| Lopinavir/ritonavir19 | ↓ | Minimal increase | Avoid combination due to HCV treatment failure. |
| Fosamprenavir/ritonavir19 | ↓ | ↓ | Avoid combination due to HIV/HCV treatment failure. |
| Darunavir/ritonavir19 | ↓ | ↓ | Avoid combination due to HIV/HCV treatment failure. |
| Atazanavir/ritonavir19 | ↓ | ↑ | Only minimal changes. Efficacy and safety being evaluated in ongoing clinical trial. |
| • Immunosuppressants | |||
| Cyclosporine23 | No effect | ↑ | 4-fold increase in cyclosporine exposure. Therapeutic drug monitoring indicated for dose optimization. |
| Tacrolimus23 | No effect | ↑ | Possible 70-fold increase in tacrolimus exposure. Avoid combination if possible. |
| Sirolimus | Unknown | ↑ | Expected increase in sirolimus exposure. Therapeutic drug monitoring indicated for dose optimization. |
| • Other Agents | |||
| Amlodipine25 | No effect | ↑ | If combination unavoidable, use lowest possible doses and titrate as tolerated. |
| Atorvastatin25 | No effect | ↑ | Avoid combination. |
| Ethinyl Estradiol26 | - | ↓ | Non-hormonal contraception should be used while taking telaprevir. |
Coadministration with antiretrovirals is concerning for both efficacy and safety of HIV and HCV therapy. Initial studies with the NNRTI efavirenz show significant decreases in telaprevir concentrations and this combination should be avoided.19 This effect is likely due to induction effects of efavirenz on CYP 3A.20 According to subsequent analysis, it was determined that higher doses of telaprevir may overcome the metabolic induction effects of efavirenz. This belief is currently being confirmed in clinical trials. When coadministered with the HIV PI’s fosamprenavir, lopinavir, and darunavir (all boosted with ritonavir), concentrations of telaprevir were significantly decreased and concentrations of fosamprenavir and darunavir were decreased.19 The mechanism of this interaction is unknown. Combination with telaprevir should be avoided to reduce failure of HIV viral suppression and HCV treatment failure. Atazanavir-based regimens are less affected than the other PI’s.19 Combination regimens of telaprevir with ritonavir-boosted atazanavir are currently under investigation in clinical trials.
It was initially believed that telaprevir could be boosted by low-dose ritonavir to maximize exposure and decrease dosing frequency. Preliminary studies in rat and human hepatic microsomes showed ritonavir significantly inhibited the metabolism of telaprevir.21 These findings were assessed in a clinical trial with healthy volunteers and it is reported that ritonavir has minimal affect on steady-state telaprevir concentrations.22 Therefore, ritonavir boosting of telaprevir is not a viable therapeutic option.
A pharmacokinetic study was identified that assessed the effect of telaprevir on cyclosporine and tacrolimus in healthy volunteers.23 For cyclosporine, pharmacokinetic parameters were mearsured after receiving a single oral dose of cyclosporine 100 mg in the absence of teleprevir. After a mandatory eight day washout period, patients received 750 mg of telaprevir every eight hours for days 1 through 11 and a 10 mg daily dose of cyclosporine was given on days 1 and 8. Through measurement of whole-blood concentrations of cyclosporine and plasma concentrations of telaprevir on days 1 and 8, pharmacokinetic profiles of both cyclosporine and teleprevir were captured. The concentrations obtained on day 8 represent steady-state concentrations of telaprevir. Dose-normalized comparisons were significantly increased for both Cmax and AUC of cyclosporine when administered with telaprevir. Cmax increased 1.3 times baseline and AUC increased 4.5 fold from baseline. The mean half-life of cyclosporine increased from 12 h at baseline to 53 h at steady state dosing, a greater than 4 fold change. Although telaprevir concentrations were obtained, samples were not taken in the absence of cyclosporine and conclusions cannot be made regarding cyclosporine’s effect on its pharmacokinetics.
For tacrolimus, in a similar fashion, healthy volunteers were given a 2 mg dose of tacrolimus for pharmacokinetic assessment in the absence of telaprevir. A minimum washout period of 14 days occurred and then patients received telaprevir 750 mg every 8 h for days 1 to 13. On day 8, representing steady-state dosing of telaprevir, a 0.5 mg dose of tacrolimus was given. Dose-normalized comparisons were significantly increased for both Cmax and AUC of tacrolimus. Cmax increased 9.3-fold and AUC increased 70-fold from baseline. The mean half-life of tacrolimus increased from 40 h at baseline to 196 h at steady state dosing. Unfortunately no baseline data was obtained for telaprevir concentrations to assess the affect of tacrolimus on its pharmacokinetics. However, given less significant inhibition of CYP 3A4 with tacrolimus as compared to cyclosporine, there is potential for less impact on teleprevir concentration.24
The results of this study confirm significant interactions exist between cyclosporine, tacrolimus and telaprevir that may expose patients to toxicity from calcineurin inhibitors. No major adverse events were recorded during the study, however, due to the minimal dose design this is not an unexpected finding.23 An extended dosing regimen, without adequate dose adjustment of calcineurin inhibitor, is likely to result in undesired effects, such as nephrotoxicity. While some are advocating that the combination of these agents should be avoided until safe dosing regimens are established,23 it is likely that these interactions will be encountered, so clinicians need to be aware and provide appropriate empiric dose adjustment of calcineurin inhibitors at initiation of PI therapy followed by very close therapeutic drug monitoring. In fact, the degree of drug interaction may require dosing of cyclosporine and tacrolimus intermittently based on drug levels. Logistics of this approach may be difficult depending on availability and turn around time for therapeutic drug monitoring assays.
A pharmacokinetic study was recently completed that assessed interactions with amlodipine and atorvastatin, both substrates of CYP3A4.25 Healthy volunteers were given single doses of amlodipine and atorvastatin at baseline and again at steady state of telaprevir. For amlodipine, the mean Cmax and AUC increased 1.27 and 2.79-fold respectively. The mean half-life increased from 41 to 95 h, which was attributed to decreases in clearance. For atorvastatin, the mean Cmax and AUC were increased 10.6 and 7.88-fold respectively. There was no statistically significant difference in half-life. Metabolites of atorvastatin also appeared to be affected by telaprevir but limited data and large variability inhibit interpretation of the results. Study investigators noted no major adverse events but this study also utilized a single dose design. The potential for serious adverse events to occur with chronic dosing has contrain-dicated the use of atorvastatin with telaprevir. Amlodipine should be avoided if possible but may be used in lower doses and titrated carefully, in order to avoid supra-therapeutic effects and toxicity.
An interaction of great therapeutic importance is coadministration with oral contraceptive pills. Ethinyl estradiol Cmax, Cmin, and AUC decreased by 26, 37, and 28% respectively when both telaprevir and ethinyl estradiol were at steady state.26 Corresponding increases in luteinizing hormone and follicle stimulating hormone were seen in relation to these changes in estrogen levels. Non-hormonal contraception should be recommended while patients are taking telaprevir, in order to prevent treatment failure.
SummarySignificant drug-drug interactions occur with both boceprevir and telaprevir. We have reviewed and discussed potential and confirmed interactions and have provided recommendations for therapy where appropriate. While boceprevir is less susceptible to metabolic interactions due to multiple pathway metabolism, clinicians need to be cautious when using this agent in combination with other agents that use or affect CYP3A. Telaprevir is a potent CYP3A inhibitor and has greater potential to cause significant drug-drug interactions. While data is available assessing some potential interactions, clinicians need to be especially careful when initiating this agent and should frequently monitor the pharmacokinetic literature to become aware of updated dosage recommendations and contraindications.
HIV patients are especially vulnerable to drug-drug interactions due to the complexity of treatment regimens and the importance of maintaining suppressed HIV-viral loads. Disruptions in therapy could compromise HIV care and result in development of resistance to antiretrovirals, exposure to undesired adverse effects, and failure of therapy. Ongoing clinical trials have been designed to help guide future treatment decisions. Until then, clinicians will need to weigh the benefits and risks of using boceprevir or telaprevir in patients taking antiretrovirals, compared to using standard therapy alone.
Transplant patients and those requiring immuno-suppression are also at high risk for clinically significant drug interactions. Tacrolimus, a first-line agent, is especially increased by telaprevir to exposure 70-fold that of regular dosing.23 Cyclosporine was also affected, although to a lesser extent. These findings have important implications for patients. Increased exposure puts patients at risk of serious and life-threatening adverse drug reactions. It is therefore essential that dose optimization studies be completed to prevent these adverse consequences. For any patient using these agents in combination, therapeutic drug monitoring of immunosuppressant levels is essential for management of care.
This review summarized the available pharmaco-kinetic literature pertaining to boceprevir and tela-previr and associated drug interactions. The major limitation of this review is the lack of published data and the reliance on unpublished data from manufacturer sources or abstracts to guide treatment decisions. Until future studies are reported and experience is gained with these agents, clinicians will need to be especially careful when administering in high-risk populations and those receiving chronic therapy with interacting agents. Studies are urgently needed in HIV patients taking antiretrovirals and patients taking chronic immunosuppresion as these populations are at increased risk of experiencing clinically significant interactions.
Conflict of InterestsIn regards to hepatitis C, Dr. Eric Yoshida has been an investigator of clinical trials sponsored by: Boeringher Ingelheim Inc., Gilead Sciences Inc., Hoffman LaRoche Inc., Human Genome Sciences, Merck Inc., Norvartis Inc., Pfizer Inc., Schering Plough Inc., Tibotec Inc., Vertex Pharmaceuticals Inc. He has received honouraria for CME lectures sponsored by Merck Inc and Hoffman LaRoche Inc. He has received honouraria for presentations given at Advisory Board Meetings of Vertex Pharmaceuticals Inc. No funds were given for the completion of this project.