Background. Approximately one-third of patients with chronic hepatitis C virus infection have persistently normal liver enzymes reflected by a normal serum alanine transaminase (ALT). Data with regards the efficacy and safety of treatment in patients chronically infected with Hepatitis C virus genotype 4 and normal serum ALT are limited. Aim. To evaluate the efficacy and safety of peginterferon alfa-2b plus ribavirin combination therapy in this population.
Material and methods. Twenty-two patients with chronic hepatitis C virus genotype 4 infection were enrolled in an open-labeled, uncontrolled pilot study. All patients had biopsy proven chronic hepatitis and persistently normal serum ALT levels. Patients were treated with subcutaneous peginterferon alfa-2b at a dose of 1.5 μg/kg body weight once per week plus oral ribavirin (15 mg/kg/day) for 48 weeks. Patients were followed for 24 weeks post-treatment.
Results. Sixteen patients out of twenty two completed the study (9 [40.9%] females, mean age 43.8 years). The ALT level were normal in all patients, with a mean of 38.6 U/L. Sustained viral response was achieved in 13 patients (59%), 4 patients (18.1%) were non-responders and 2 patients (9%) relapsed while 1 patient had a viral breakthrough during treatment. Two patients (9%) discontinued the treatment because of adverse events.
Conclusions. Combination therapy of pegylated interferon-alpha2b and ribavirin is safe and resulted in a sustained viro-logical response in a significant number of patients with chronic Hepatitis C, genotype 4, and persistently normal serum ALT.
Chronic hepatitis C virus (HCV) infection is the leading cause of liver disease in the Middle East with a prevalence of up to 20% in some populations.1–3 The most common genotype in the region is genotype 4.4,5 Most patients chronically infected with HCV have elevated serum aminotransferase levels, while approximately 25-40% of the infected patients exhibit normal aminotransferase levels.6–8 These patients are usually not given anti-HCV treatment, as majority of them have mild degree of histological damage on liver biopsy.9–13 The natural clinical course of these patients is not well understood.9–11,14 Some studies report that approximately 30% of these subjects may progress to cirrhosis, and the development of hepatocellular carcinoma has also been described in some cases.11,13 These observations raise the question of treatment of these patients, particularly with the availability of the new antiviral therapy.
The development of direct acting antiviral (DAA) agents such as boceprevir and telaprevir in recent years has ushered in a new era in the therapy of HCV.15,16 However these agents are currently only approved for use in patients with HCV genotype 1 infection. To the best of our knowledge there are currently no clinical trials of the use of direct acting antiviral agents in HCV genotype 4 infection and we are unaware of any DAA agents in development that are specific for HCV genotype 4. Therefore, in these patients, peginterferon and ribavirin will remain the only available antiviral therapy in the foreseeable future.
ObjectiveThe aim of this study was to determine the efficacy and safety of pegylated interferon alfa-2b and ribavirin combination therapy in chronically infected patients with HCV genotype 4 who have persistently normal serum transaminases.
Material and MethodsPatients selectionTreatment-naïve patients infected with HCV genotype 4 and persistently normal alanine transa-minase (ALT) levels were eligible for inclusion in the study. The patients were included independent of mode of acquisition of infection, or level of serum HCV RNA level. The criteria for inclusion in the study were:
- •
Age between 16 and 65 years.
- •
HCV RNA detectable by polymerase chain reaction.
- •
Infection with HCV genotype 4.
- •
Liver biopsy findings consistent with the diagnosis of chronic hepatitis C.
- •
Compensated liver disease (Child-Turcotte class A), and
- •
Persistently normal serum ALT level, defined as normal ALT values at regular 2 months interval over a six-month period.
Criteria for exclusion were:
- •
Clinical evidence of hepatic decompensation, e.g. presence of ascites, hepatic encephalopathy, vari-ceal hemorrhage.
- •
Serum albumin < 30 g/L; total serum bilirubin > 50 μmol/L; prothrombin time > 4 s above control.
- •
Serum creatinine > 140 mmol/L.
- •
Hemoglobin < 110 g/L.
- •
Total leukocyte count < 2 x 109/L.
- •
Platelets < 50 x 109/L.
- •
Seropositivity for hepatitis B surface antigen or human immunodeficiency virus.
- •
Serum iron saturation 150%.
- •
Alcohol or drug dependence.
- •
Serious comorbid conditions.
- •
Severe psychiatric disorders, and
- •
Treatment with anti-viral or immunosuppressive agents prior to enrollment.
All women of childbearing potential were required to have a negative pregnancy test at the time of inclusion in the study.
Serum HCV RNA testing was performed using a qualitative polymerase chain reaction assay which has a lower limit of detection of 50 IU/mL (Cobas Amplicor HCV Monitor Test, Version 2.0, Roche Diagnostics, Branchburg, New Jersey). HCV geno-typing was performed using the Versant HCV Genotyping assay (LiPA) (Innogenetics, Ghent, Belgium). Patients having a HCV RNA of ≤ 600,000 IU/ mL were considered to have a low viral load; while those with HCV RNA of > 600,000 IU/mL were considered to have a high viral load. All patients underwent a liver biopsy prior to initiation of anti-HCV therapy and all biopsies were reviewed by a single pathologist scored using the METAVIR system.17
The study was conducted in accordance with the Declaration of Helsinki, and the protocol and the statement of informed consent were approved by the ethical committee of the Medical Research Council, Faculty of Medicine, Kuwait University. All patients gave an informed consent prior to inclusion in the study.
Study designThis was an open-label uncontrolled pilot study. The treatment consisted of peginterferon alfa-2b (Pe-gintron®, Schering-Plough Corp., Kenilworth, New Jersey) at a dose of 1.5 μg/kg body weight per week, subcutaneously in combination with ribavirin (Re-betol®, Schering-Plough Corp., Kenilworth, New Jersey) 15 mg/kg/day for 48 weeks. Ribavirin was administered orally with food in two or three divided doses daily. Any patient who received a single dose of treatment was included in the analysis.
MonitoringThe patients were evaluated 2 weeks after starting the treatment and then monitored every 4 weeks during the entire 48-week treatment period. Evaluation at each visit consisted of clinical assessment, complete blood and reticulocyte counts, as well as liver and renal profiles. Serologic tests for thyroid stimulating hormone, and quantitative HCV RNA by PCR were obtained at study weeks 12, 24 and 48 of treatment. The patients were monitored for additional 24 weeks after completion of therapy. A quantitative HCV RNA by PCR was obtained at the end of this period at week 72.
Assessment of efficacyPatients who had a undetectable HCV RNA (defined as < 50 IU/mL) at treatment week 12 were considered to have achieved a complete early virological response [C-EVR], while those who has ≥ 2 log reduction in HCV RNA level compared to baseline HCV RNA level) were considered to have achieved a partial early virological response (P-EVR). Patients who failed to clear HCV RNA from serum after 24 weeks of therapy were considered as non-responders and did not receive further treatment. Those patients who had C-EVR or P-EVR continued treatment until week 48. Patients who had undetectable HCV RNA negative at the end of week 48 were considered to have end-of-treatment response (ETR). Those patients who had reappearance of HCV RNA in serum while still on therapy were considered to have viral breakthrough. All patients who achieved ETR after 48 weeks were followed for an additional 24 weeks after finishing treatment. Those patients who had an undetectable HCV RNA at week 72 were determined to have achieved sustained virological response (SVR), while those in whom there was reappearance of HCV RNA in serum after discontinuation of therapy were labeled as having had a relapse.
The primary efficacy end point was SVR. Secondary end points include C-EVR and ETR at week 48.
Compliance monitoringPeginterferon alfa-2b was administered to all patients in a local primary care clinic by a registered nurse who documented compliance. Adherence to ri-bavirin ingestion was monitored by capsule count.
Statistical analysisThe data were analyzed using the SPSS software (SPSS Inc., Chicago, IL, USA). We used the Z-test to compare the difference between two proportions means. A p-value of < 0.05 was considered statistically significant. All p-values presented are two-sided.
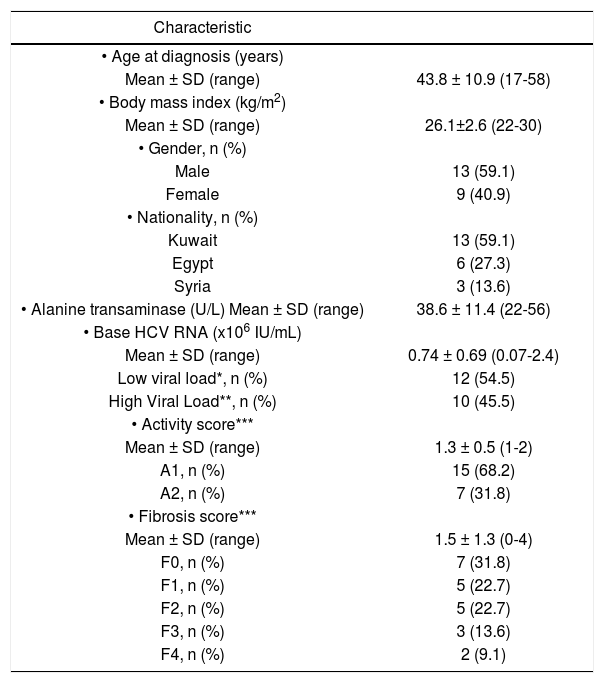
ResultsTable 1 shows the socio-demographic characteristics of the 22 patients (13 males, 9 females) enrolled in the study. The mean age at the time of inclusion in the study was 43.8 ± 10.9 (range 17-58) years. All 22 patients were Arabs, of whom 13 (59.1%) were Kuwaiti nationals, 6 (27.3%) Egyptians and 3 (13.6%) Syrians. The mean body mass index (BMI) was 26.1 ± 2.6. A total of 12 (54.5%) patients were overweight (BMI 25.0-29.9) and 3 (13.6%) were obese (BMI > 30). The mean ALT level prior to initiation of therapy was 38.6 ± 11.4 (range 22-56). HCV RNA was detected in the baseline sera of all patients with a median level of 0.74 ± 0.69 x 106 IU/mL (range 0.07-2.4 x 106), 12 (54.5%) patients had low viral load (LVL) while 10 (45.4%) had high viral load (HVL). The pre-treatment liver biopsy showed disease activity scores of A1 in 15 (68.2%) and A2 in 7 (31.8%) patients. Seventeen (77.3%) patients had a fibrosis score of ≤ F2, while 7 (31.8%) patients had a fibrosis score of > F2.
Characteristics of 22 patients with HCV genotype 4 with normal ALT treated with weight-based pegylated interfe-ron-alpha2b plus ribavirin.
| Characteristic | |
|---|---|
| • Age at diagnosis (years) | |
| Mean ± SD (range) | 43.8 ± 10.9 (17-58) |
| • Body mass index (kg/m2) | |
| Mean ± SD (range) | 26.1±2.6 (22-30) |
| • Gender, n (%) | |
| Male | 13 (59.1) |
| Female | 9 (40.9) |
| • Nationality, n (%) | |
| Kuwait | 13 (59.1) |
| Egypt | 6 (27.3) |
| Syria | 3 (13.6) |
| • Alanine transaminase (U/L) Mean ± SD (range) | 38.6 ± 11.4 (22-56) |
| • Base HCV RNA (x106 IU/mL) | |
| Mean ± SD (range) | 0.74 ± 0.69 (0.07-2.4) |
| Low viral load*, n (%) | 12 (54.5) |
| High Viral Load**, n (%) | 10 (45.5) |
| • Activity score*** | |
| Mean ± SD (range) | 1.3 ± 0.5 (1-2) |
| A1, n (%) | 15 (68.2) |
| A2, n (%) | 7 (31.8) |
| • Fibrosis score*** | |
| Mean ± SD (range) | 1.5 ± 1.3 (0-4) |
| F0, n (%) | 7 (31.8) |
| F1, n (%) | 5 (22.7) |
| F2, n (%) | 5 (22.7) |
| F3, n (%) | 3 (13.6) |
| F4, n (%) | 2 (9.1) |
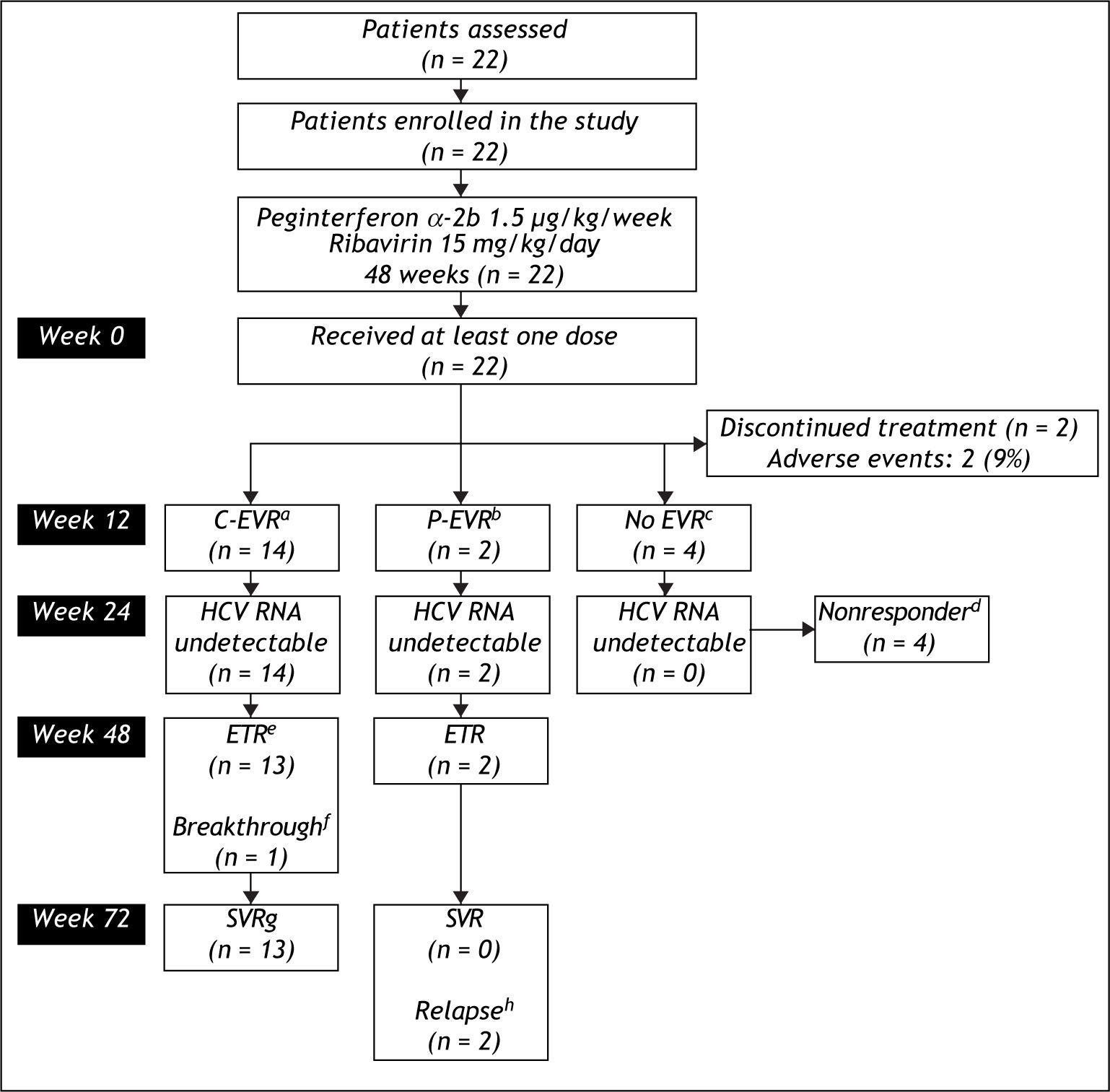
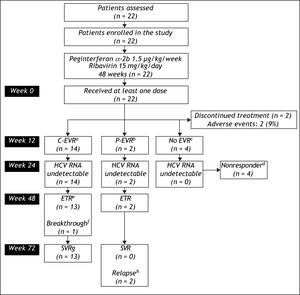
Figure 1 shows the flow of patients through the study. Two (9.1%) patients withdrew from study within the first 12 weeks because of adverse effects from the medications. The remaining 20 patients received at least 80% of the peginterferon alfa-2b and ribavirin. At the end of week 12 of treatment a total of 14 (63.6%) patients achieved C-EVR, while 2 (9.1%) achieved P-EVR. There were 4 (18.2%) patients who failed to achieve EVR were declared non-responders and received no further treatment.
Flow of patients through the study.
aC-EVR: complete early viro-logical response, defined a completed loss of HCV RNA after 12 weeks of therapy.
bP-EVR: partial early virological response, defined a ≥ 2 log decrease in, but not complete absence of, HCV RNA after 12 weeks of the-rapy.
cEVR: early virological response.
dNonresponder: Failure to cleare HCV RNA from serum after 24 weeks of therapy.
eETR: end-of-treatment response, defined as HCV RNA negative by a sensitive test at the end of 48 weeks of treatment.
fBreakthrough: reappearance of HCV RNA in serum while still on therapy.
gSVR: sustained virological response, defined as HCV RNA negative 24 weeks after cessation of treatment.
hRelapse: reappearance of HCV RNA in serum after therapy is discontinued.
The secondary endpoint of ETR was achieved in 15 (68.2%) patients, who included 13 (92.8%) patients who had achieved C-EVR and both the patients with P-EVR. One (4.5%) patient who had achieved C-EVR had detectable HCV RNA in his serum at week 48 (breakthrough). A total of 13 (59%) patients achieved the primary endpoint of achieving SVR. All 13 patients who had SVR were those who had achieved C-EVR. Both the patients who had P-EVR but had reached ETR relapsed by week 72 and failed to achieve SVR. None of these patients experienced a flare-up in ALT levels during or after the cessation of treatment. The mean ALT in the 13 patients who achieved SVR decreased from 38.8 ± 11.3 IU/L to 28.0 ± 5.0 IU/L at week 72.
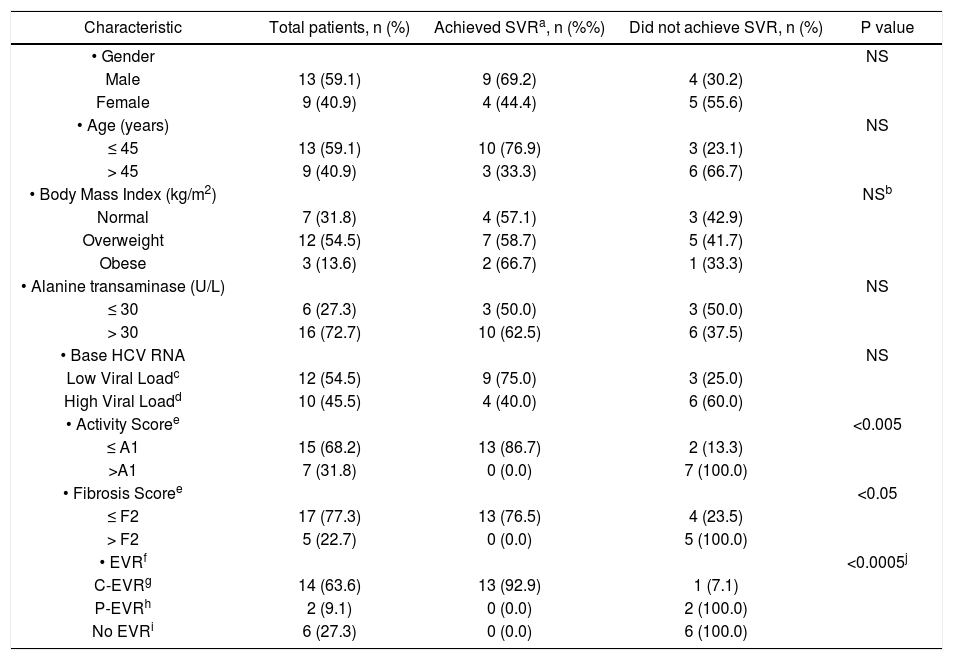
Table 2 compares the characteristics of patients who either achieved or failed to reach SVR (including the 2 patients who discontinued treatment within 12 weeks because of adverse events). The proportion of male patients who achieved SVR was 69.2% compared to 44.4% of the females. 76.9% of the patients aged ≤ 45 years achieved SVR compared to 33.3% of those who were older than 45 years. The proportion of patients with normal BMI who reached SVR was 57.1% compared to 58.7% of those who were overweight and 66.7% of obese patients. Patients who had a baseline ALT of ≤ 30 U/L achieved SVR in 50.0% compared to 62.5% of those who had a base ALT of > 30 U/L. However, none of the above differences were found to be statistically significant (p > 0.05).
Predictors of response in 22 patients with HCV genotype 4 with normal ALT treated with weight-based pegylated inter-feron-alpha2b plus ribavirin.
| Characteristic | Total patients, n (%) | Achieved SVRa, n (%%) | Did not achieve SVR, n (%) | P value |
|---|---|---|---|---|
| • Gender | NS | |||
| Male | 13 (59.1) | 9 (69.2) | 4 (30.2) | |
| Female | 9 (40.9) | 4 (44.4) | 5 (55.6) | |
| • Age (years) | NS | |||
| ≤ 45 | 13 (59.1) | 10 (76.9) | 3 (23.1) | |
| > 45 | 9 (40.9) | 3 (33.3) | 6 (66.7) | |
| • Body Mass Index (kg/m2) | NSb | |||
| Normal | 7 (31.8) | 4 (57.1) | 3 (42.9) | |
| Overweight | 12 (54.5) | 7 (58.7) | 5 (41.7) | |
| Obese | 3 (13.6) | 2 (66.7) | 1 (33.3) | |
| • Alanine transaminase (U/L) | NS | |||
| ≤ 30 | 6 (27.3) | 3 (50.0) | 3 (50.0) | |
| > 30 | 16 (72.7) | 10 (62.5) | 6 (37.5) | |
| • Base HCV RNA | NS | |||
| Low Viral Loadc | 12 (54.5) | 9 (75.0) | 3 (25.0) | |
| High Viral Loadd | 10 (45.5) | 4 (40.0) | 6 (60.0) | |
| • Activity Scoree | <0.005 | |||
| ≤ A1 | 15 (68.2) | 13 (86.7) | 2 (13.3) | |
| >A1 | 7 (31.8) | 0 (0.0) | 7 (100.0) | |
| • Fibrosis Scoree | <0.05 | |||
| ≤ F2 | 17 (77.3) | 13 (76.5) | 4 (23.5) | |
| > F2 | 5 (22.7) | 0 (0.0) | 5 (100.0) | |
| • EVRf | <0.0005j | |||
| C-EVRg | 14 (63.6) | 13 (92.9) | 1 (7.1) | |
| P-EVRh | 2 (9.1) | 0 (0.0) | 2 (100.0) | |
| No EVRi | 6 (27.3) | 0 (0.0) | 6 (100.0) |
The proportion of patients with a baseline LVL (HCV RNA of ≤ 600,000 IU/mL) and reached SVR was 75% compared to 40.0% of those with a HVL. However, this difference did not reach statistical significant (p > 0.05). A majority of patients (86.7%) who had a necro-inflammatory activity score on liver biopsy of ≤ A1 achieved SVR compared to none of those who had an activity score of > A1 (p < 0.005). Similarly, 76.5% of those patients who had a fibrosis score of ≤ F2 on the liver biopsy reached SVR compared to none of those with a fibrosis score of > F2 (p < 0.05). A total of 13 (92.9%) patients who had C-EVR reached SVR, compared to none of the 2 patients who had P-EVR (p < 0.05), or the 6 patients who did not have EVR (p < 0.005).
DiscussionPersistently normal ALT (PNALT) is usually defined as ALT levels in the normal range on at least 4 different occasions 3 months apart over a twelve months period. The need for treatment in this group of patients has been controversial. Several studies have shown that the progression of fibrosis in this subset of patients is slow and the natural history of these healthy carriers appears to be benign.9,18,19 Besides this, the many side effects associated with in-terferon therapy along with its high cost and the reports of very low SVR from studies with interferon monotherapy caused many experts to recommend against the treatment of these patients. More recent studies have reported target organ liver damage including cirrhosis in patients with chronic HCV infection and persistently normal ALT levels.11,13,20 There is no reliable method to predict which patients are at risk of progression to severe liver disease. Sudden worsening of disease with ALT increase, histological deterioration and in some cases even development of HCC has been described after 15 years of follow up.21–24 From a public health perspective, these patients with normal ALT may serve as a reservoir for the virus in the community and thus treatment could be considered to eliminate this reservoir of infection. A French study has shown that treatment of PNALT patients in the same manner as elevated ALT (EALT) patients will reduce the mortality and morbidity related to hepatitis C. These would strongly argue in favor of treating chronic hepatitis C patients with PNALT.
The combination of pegylated interferon and ri-bavirin is at present the treatment of choice in patients with chronic HCV and EALT and so it is presumed to be the optimal therapy for patients with PNALT as well. The data regarding the efficacy and safety of antiviral therapy in patients with genotype 4 infection and normal ALT are limited since most of the therapeutic trials have enrolled HCV infected patients with elevated serum ALT levels. In the limited studies available, the safety profile in PNALT patients was similar to that in patients with elevated ALT levels receiving pegin-terferon plus ribavirin.
The randomized trials with pegylated interferon in genotype 1 showed comparable SVR in patients with PNALT and EALT levels.25,26 Though SVR rates were much lower in the studies with regular in-terferon and ribavirin when compared to those with pegylated interferon and ribavirin, the SVR in PNALT group were similar to those of EALT group in the studies with genotype 4 chronic hepatitis C patients.27 The SVR of 59% in genotype 4 HCV positive patients with PNALT in our study is comparable to the SVR of 44-69% in HCV positive genotype 4 patients with raised liver enzymes.28–31 This is also similar to the SVR of 56% seen in a study on genotype 4 patients with PNALT using pegylated interfe-ron alpha-2a and ribavirin. The EVR and ETR were also similar to those found in other studies with genotype 4 patients with raised ALT.28,30–33
There were no ALT flares during interferon treatment in our study unlike that observed in some earlier studies.34 This finding is in agreement with studies where ALT flares were not noted during the treatment. The side effects were not significantly higher in our study in comparison to other studies on genotype 4 with EALT.28,30 In patients who achieved SVR the mean ALT dropped by 10 U/L after treatment compared with pretreatment values. This suggests that the normal values in these patients may be higher than their true normal values and the viral clearance with associated improvement in hepatic inflammation resulted in regression of these values to their true normal. This may mean that the currently accepted ULN for serum AST and ALT levels are too high. Some researchers have attempted to re-evaluate the ULN for serum ALT in chronic hepatitis C in order to redefine normal serum ALT levels.35–37 This also suggests that ALT activity may be an unreliable guide for treatment decisions and indeed, treatment of patients with chronic hepatitis C should rely on the probability of viral eradication, symptoms, histology, anticipated progression of disease, and the risk of transmission rather than on a single biochemical parameter. Nearly 45% of the patients in our study had significant fi-brosis (i.e. F2 or higher) and 9% had cirrhosis on liver biopsy. The factors which were the most significant predictors of SVR in our patients included attainment of C-EVR, and presence of an activity score of ≤ A1 and fibrosis scores of ≤ F2 on liver biopsy. Therefore, neither the presence of PNALT, nor a fibrosis score of up to F2 on liver biopsy should routinely exclude patients with HCV genotype 4 from receiving combination therapy with pe-gylated interferon and ribavirin.
Though it is a nonrandomized, open label study with only a small number of patients the study is relevant because of the lack of studies reported in the literature on pegylated interferon alpha 2b in HCV genotype 4 with PNALT. The findings from the study suggest that the response rate and safety profile of this treatment in PNALT patients may be comparable to EALT patients with genotype 4.
ConclusionOur pilot study has shown that pegylated interfe-ron alpha 2b plus ribavirin is an effective and safe treatment for chronic HCV genotype 4 infection with PNALT, and that ALT levels should not be the sole determinant in deciding the need for treatment in chronic HCV infection. However as this is a pilot study, the findings needs to be confirmed by further randomized and bigger trials.
Abbreviations- •
HCV: Hepatitis C virus.
- •
ALT: Alanine aminotransferase.
- •
RNA: Ribonucleic acid.
- •
PCR: Polymerase chain reaction.
- •
SVR: Sustained virological response.
- •
EVR: Early virological response.
- •
ETR: End-of-treatment response.