Acute hepatitis E is becoming increasingly recognised in Europe with up to 40% of the population in Southern France being exposed to the virus, which is harboured in pigs. Patients with known liver disease may present with acute hepatitis E and present a diagnostic challenge. For example patients with autoimmune hepatitis (AIH) who are immunosuppressed and contract hepatitis E may be at increased risk of developing chronicity due to concurrent immunosuppression. Importantly, the diagnosis may be missed with the infection misdiagnosed as an autoimmune flare, and immunosuppression increased by the attending physician, thus enhancing the risk of chronicity of infection leading to progressive liver injury in immunocompromised patients.
We report a case of acute hepatitis E in a patient with AIH and discuss the features that helped us differentiating it from an autoimmune flare.
Recent studies suggest that autochthonous hepatitis E in industrialised countries may be more common than previously believed, as reflected by the high hepatitis E immunoglobulin G (IgG) seroprevalence rates reported in Europe and in the United States.1-3 The vast majority of these infections are asymptomatic and usually go unrecog-nised.4 In addition, it is likely that symptomatic hepatitis due to hepatitis E virus (HEV) is frequently misdiagnosed in clinical practice. For instance, a retrospective serologi-cal study in the UK found that a relevant proportion of cases of hepatitis originally labelled as “drug-induced liver injury” were actually due to HEV infection.5 In general, a high index of suspicion is needed to make a diagnosis of autochthonous hepatitis E. This is particularly true in the setting of pre-existing chronic liver diseases causing recurrent elevations in transaminases levels, such as autoimmune hepatitis (AIH). In fact, in the context of chronic AIH, it is even more important to make a correct diagnosis of acute hepatitis E infection because management of these two conditions is different. Intensification in immunosuppressive therapy is used to control hepatitis flares which are autoimmune in nature.6 Persistence of HEV following an acute infection and progressive HEV-induced liver damage has been reported in immunocompromised patients, such as solid organ transplant recipients, patients with HIV/AIDS and patients with haematological diseases receiving chemotherapy and bone marrow transplant re-cipients.7-11 It can be speculated that potent immunosup-pression may lead to a more prolonged and severe course of hepatitis E infection in patients with AIH.
We describe a case of acute hepatitis E in a patient with a 12-year history of AIH and highlight the elements that helped us to differentiate this infection from an autoimmune flare.
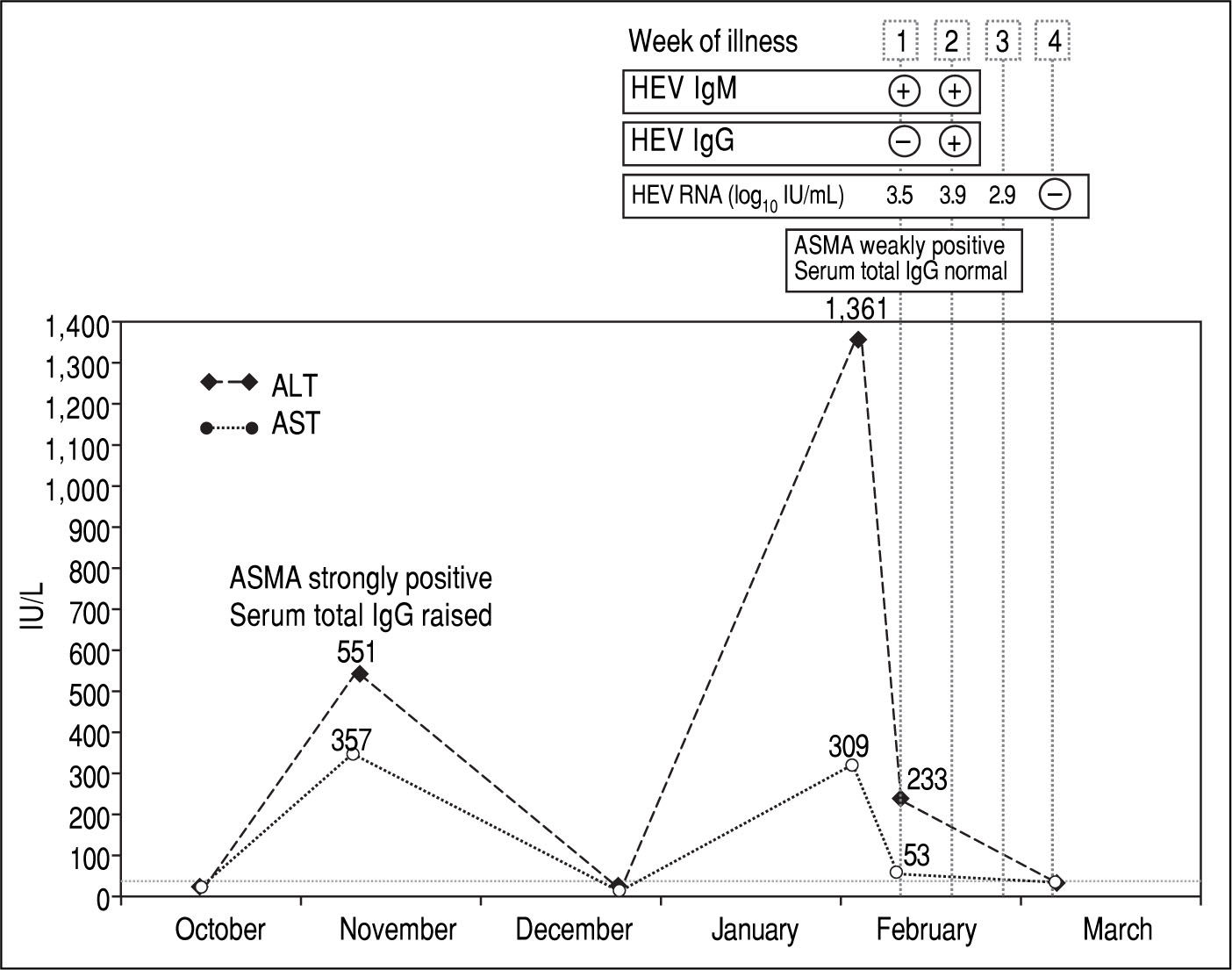
Case ReportA 44-year-old British woman with chronic AIH was noted to have ALT 551 IU/L [normal range 5-33 IU/L], AST 357 IU/L [normal range 5-31 IU/L], a strongly positive anti-smooth muscle antibody (ASMA) titre and raised total serum IgG (19.4 g/L, normal range 7-16 g/L) on a routine follow-up appointment in the Hepatology Clinic in November 2014 (Figure 1). She had been diagnosed with AIH 12 years previously and her diagnosis met the criteria set by the International Autoimmune Hepatitis Group (IAIHG) for definite AIH with a composite score of 7.12 On that appointment, her prednisolone dose was increased from 5 to 30 mg/day and azathioprine 75 mg/day was added.
Five weeks later, her liver enzymes had returned to normal levels and her dose of prednisolone was reduced to 20 mg/day with a planned reduction to 10 mg over the next few weeks. In January 2015, she went on holiday to Spain for five days.
At a subsequent clinic visit one month after the holiday in Spain, the patient complained of tiredness, general malaise and night sweats that had started seven days before. Physical examination was unremarkable. Her predniso-lone dose had been increased by her General Practitioner five days previously, as a blood test done one week earlier showed that the alanine aminotransferase (ALT) had increased from normal to 1,361 IU/L. Blood tests repeated in clinic confirmed a “hepatitis flare” with ALT at 233 IU/ L and aspartate aminotransferase (AST) at 53 IU/L and ASMA weakly positive. Total serum IgG, bilirubin, alkaline phosphatase, full blood count, renal function tests, serum electrolytes and coagulation profile were all within normal limits. Results of a viral hepatitis screen were negative for hepatitis A IgM, hepatitis B surface antigen and hepatitis C IgG. However, HEV IgM was positive, with negative HEV IgG, and HEV RNA was detected in blood at 3.5 log10 IU/mL and identified on sequencing as genotype 3c. HEV antibody testing was performed using the recomWell and recomLine assays (Mikrogen, Neu-ried, Germany) in accordance with the manufacturer's instructions. HEV RNA was detected with an internally controlled RT-PCR with a detection limit as defined by Poisson titration of 22 IU/mL.13 Plasma RNA was amplified, sequenced, and subjected to genotypic analysis across part of the open reading frame 2 as previously described.14
A diagnosis of acute hepatitis E was made and, in order to minimise the potential risk of progression to chronic infection, azathioprine was temporarily stopped and pred-nisolone was rapidly reduced to 7.5 mg/day. Two weeks after onset of symptoms, the patient seroconverted for HEV IgG and HEV RNA was still detected at 3.9 log10 IU/ mL. By the end of the third week of illness, the HEV viral load dropped to 2.9 log10 IU/mL. Testing repeated a week later showed undetectable HEV RNA, AST and ALT levels within normal range and her symptoms had completely resolved.
DiscussionNo previous stored samples were available for this patient, so retrospective hepatitis E testing could not be performed. In the absence of stored samples, it is difficult to establish with confidence whether hepatitis E had persisted for a significant period of time and her hepatic illness was triggered by the gradual reduction of the steroids in December that initiated a host attempt to clear the virus, or whether this was a recently acquired acute hepatitis E occurring in the setting of pre-existing chronic liver disease. Comparison of the two episodes of transaminitis, however, reveals several differences, which provide clues to their aetiology. In November 2014, AST and ALT increased to a similar degree, whereas in February 2015 AST increased to a much lesser degree compared to ALT. ASMA was strongly positive and total serum IgG was raised in November 2014, whereas in February 2015 ASMA was weakly positive and total serum IgG was within the normal range. Finally, the first episode of transaminitis occurred when the level of immunosuppres-sion was relatively low, and improved when immunosup-pressive treatment was increased, whereas the second episode manifested whilst the patient was receiving intensified immunosuppression. These findings, together with the observation that that the patient seroconverted for HEV IgG in February 2015, suggest that it is more likely that the patient had a flare of her autoimmune hepatitis in November 2014 followed by an acute hepatitis E infection in February 2015.
There was no history of acute hepatitis in any household or family contacts. Upon further questioning, the patient reported having eaten cooked pork sausages and other pork products on several occasions in Spain as well as in the UK in the 90 day-period preceding her presentation in February 2015. Although it cannot be established with certainty, it seems more likely that the patient acquired the infection in the UK. Most infections in Spain are due to HEV genotype 3f, whereas the strain detected in our patient was 3d (15). Furthermore, the top ten BLAST hits against the NCBI GenBank nucleotide sequence database of the strain detected in our patient were with seven UK sequences and three Dutch sequences.
It is now well-established that HEV infection can persist in individuals with impaired antiviral immune defenc-es.4 Chronic hepatitis E has been extensively described in solid organ transplant patients, HIV-infected patients with low (< 200/mm3) CD4 counts and patients with haemato-logical malignancies.16 Even though persistence of HEV infection beyond these well-established risk groups is uncommon, there are case reports of chronic hepatitis E in patients with impaired immunity due to other causes, such as granulomatosis with polyangitis treated with mycophe-nolate and prednisolone therapy, retroperitoneal fibrosis treated with sirolimus and prednisolone and systemic lupus erythematous.17,18 To our knowledge, there is only one case report published to date describing the occurrence of chronic hepatitis E in the background AIH.17 Interestingly, the patient described by Höner zu Siederdissen, et al. had not received any immunosuppressive therapy for his AIH, however, immunological tests revealed an underlying undefined CD4 T-cell defect.17
A few years ago, Pischke, et al. reported an increased HEV IgG seroprevalence among patients with AIH compared to healthy controls in Germany and speculated that HEV may act as a trigger for AIH in a subset of patients and that some cases of AIH may actually be misdiagnosed HEV infections.19 A subsequent larger study conducted in the Netherlands using the Wantai (Beijing, China) assay, which is the most sensitive assay currently available for HEV serology, did not confirm but could not rule out either Pischke's findings.20 More and larger studies are needed to investigate the possibility of an association between these two conditions and its nature. Our patient's diagnosis of AIH was definite, according to the IAIHG criteria, and was made 12 years prior to HEV IgG seroconversion, so we think that neither of the two possible pathogenetic relations suggested by Pischke could apply to our patient.
Although there is no evidence showing that a reduction in immunosuppressive therapy influences the risk of progression to chronicity following HEV infection, it has been shown that this approach can lead to HEV clearance in up to one-third of transplant patients who have already developed chronic infection.21 Our decision to stop azathioprine and reduce the dose of pred-nisolone was based on this indirect evidence.21
In conclusion, there is a now strong body of evidence to indicate that hepatitis E should be included in the differential diagnosis in all cases of unexplained acute hepatitis in western countries, regardless of travel history. The case we describe suggests that also in patients with AIH testing for hepatitis E should be considered every time the clinical and biochemical picture does not seem to fit completely with an autoimmune flare.
Abbreviations- •
AIH: autoimmune hepatitis.
- •
ALT: alanine aminotransferase.
- •
ASMA: anti-smooth muscle antibody.
- •
AST: aspartate aminotransferase.
- •
HEV: hepatitis E virus.
- •
IgG: Immunoglobulin G.
No funding source to declare.
Conflict of InterestNo potential competing interests to declare.