Hepatocellular carcinoma (HCC) is the sixth most common cancer in the world in terms of incidence, accounting for approximately 630 thousand new cases per year; in addition, HCC is the third most common cause of cancer death. Worldwide, the greatest risk factors for HCC are the infections caused by hepatitis B and C viruses, which increase the risk of developing the disease by about 20 times. The standard treatment in the early stages of the disease, such as surgical resection, local ablation and liver transplantation, are able to cure a proportion of patients, but most cases of HCC present in advanced stages, precluding the use of such treatments with curative intent. In these advanced stages, systemic treatments are commonly used. Unfortunately, chemotherapy with conventional cytotoxic agents is ineffective and does not seem to modify the natural history of disease. Treatment options for patients with advanced HCC are extremely limited, but the identification of signaling pathways, and the recognition of the role of these pathways in the pathogenesis of the disease resulted in the development of drugs directed at specific therapeutic targets. One such drug is Sorafenib, a kinase inhibitor with antiangiogenic and antiproliferative properties. In conclusion, Sorafenib has demonstrated survival benefits in patients with advanced HCC, thus representing a new standard reference for systemic treatment in these cases.
Hepatocellular carcinoma (HCC) is among the most frequent malignant tumors in adults, witha high incidence in some countries, especially in the Asian continent. Moreover, its incidence has been increasing over the past decades in many countries, including Brazil, chiefly due to the increased prevalence of chronic infection with hepatitis B (HBV) and C viruses (HCV), which are currently the main cause of HCC. Patients with HCC usually present with tumors in advanced and incurable stages. Until recently, the systemic treatment for these patients was very toxic and not very effective, but this situation is changing with a better understanding of the molecular mechanisms involved in the pathogenesis of HCC. Currently, the development of treatments based on molecular targets, such as sorafenib, has the greatest potential to modify the natural history of advanced HCC. This article aims to review the mechanisms of hepatocarcinogenesis and the role of sorafenib in the treatment of patients with advanced HCC.
EpidemiologyHCC is the sixth most common type of cancer in terms of incidence, accounting for approximately 630,000 new cases per year in the world; in addition, HCC is the third leading cause of cancer deaths.1 Worldwide, the biggest risk factors for HCC are the infections caused by HBV and HCV, which increase the risk of developing liver cancer by about 20-fold.2 Thus, the incidence of HCC is directly related to the prevalence of chronic infections caused by HBV and HCV. Around 80% of HCC cases occur in developing countries, with the areas of high incidence being the sub-Saharan Africa, East and Southeast Asia, and Melanesia. On the other hand, the incidence of HCC is low in many developed countries, as well as in Latin America and South-central Asia.1
In Latin America, the epidemiological burden of HCC is likely to be underestimated, since no reliable data are available and because the local literature on HCC incidence and mortality is relatively scarce. However, a recent prospective, multicenter, international, observational study organized by the Latin American Association for the Study of the Liver was published. This important report showed results that are similar to those from other Western countries, in the sense that HCC emerges on a background of underlying cirrhosis in 85% of cases; furthermore, it has been noted that HCV is the more frequent cause of HCC in this area (38%).3 A few retrospective studies have been performed in Latin American countries; one such study has found that in Mexico the mortality from HCC is trending upwards. This study has also demonstrated that the mortality was higher in men than in women in the evaluation period (2000-2006).4
In Brazil, the Ministry of Health estimates that at least 15% of the population has had contact with HBV, and that the number of cases of chronic hepatitis B and C should correspond to around 1.0% or 1.5% of the population, respectively.5 Despite these figures, the incidence of HCC in Brazil is unknown, since the disease is not in the list of the 10 most frequent malignancies according to the Brazilian National Cancer Institute.6 Taking into account that there is a high prevalence of HBV and HCV infections in Brazil, it is possible that HCC is under-diagnosed in this country. Furthermore, it is likely that an increase in the number of HCC cases will occur over the next few decades, due to the constant increase in the prevalence of hepatitis B and C in Brazil.
Despite the absence of national data on the incidence of HCC, a study conducted in Brazil pictured the profile of patients with this tumor in eight states. The mean patient age was 56.3 years for males and 54.7 years for females, with a ratio between male and female patients with HCC of 3.4 to 1. Moreover, the hepatitis-B virus surface antigen, which indicates active infection with this virus, was positive in 41.6% of cases, while the antibody against hepatitis C virus was found in 26.9% of patients. Chronic alcoholism was reported in 37% of patients. HCC was found in cirrhotic livers in 71.2% of the cases in which the presence or absence of cirrhosis was reported.7
PathogenesisAll etiological factors associated with HCC are causes of chronic liver disease. Thus, cirrhosis is the true risk factor for tumor development, since nearly 80% of patients with HCC pass through the stage of cirrhosis before developing the tumor.8 In population terms, the three main causes of HCC are hepatitis viruses (HBV and HCV), and alcoholism. Among the rarer causes, mainly in African and Asian countries, are included the aflatoxins –particularly aflatoxin B1–, which are carcinogenic substances produced by fungi of the genus Aspergillus that can colonize grains such as soybeans, peanuts, and corn. Finally, some autoimmune and metabolic diseases and, possibly, hepatic steatosis, may be associated with HCC in some cases.9 The mechanisms by which these etiological factors lead to cirrhosis and development of HCC may involve chronic inflammation, which has been progressively recognized as a pro-carcinogenic condition.1011
Hepatocarcinogenesis is a multifactorial process in which external stimuli induce genetic changes in mature hepatocytes leading to proliferation and cell death.12 The genetic alterations that accumulate in chronic hepatitis and cirrhosis after repeated defects and regeneration of hepatocytes directly contribute to hepatocarcionogenesis, with aneuploidy being the most common alteration. HCC tumor cells are characterized by a considerable loss of heterozygosity that includes multiple chromosomes; in addition, mutations are found in several important genes, including p73, p53, Rb, APC, DLC-1 (deleted in liver cancer), p16, PTEN, IGF-2, BRCA2, SOCS-1, Smad2 and Smad4, β-catenin, c-myc, and cyclin D1.13,14 Among the molecular mechanisms and signaling pathways involved in the development of HCC that regulate cell proliferation and survival, growth factor receptors, growth factors themselves, angiogenic factors, several oncogenes and intracellular signaling pathways stand out. The expression of epidermal growth factor (EGF) and other family members, such as transforming growth factor alpha and heparin-binding epidermal growth factor, as well as the EGF receptor (EGFR), have been described in several cell lines and in HCC tissue.12 Angiogenic factors such as vascular endothelial growth factor (VEGF), the platelet-derived growth factor receptor (PDGFR), and fibroblast growth factor, are released by the tumor; moreover, inflammatory cells and tumor stromal cells participate in HCC neovascularization.15
As in other malignancies, HCC is characterized by an imbalance in growth promoting and regulation signals, and the mitogen-activated protein kinase (MAPK) cascade is signaling pathway that has undergone more extensive characterization in this type of cancer.1216 This signaling pathway is activated when a growth factor binds to specific receptors located on the membrane of target cells. Next, there is autophosphorylation of the receptor, and the cytosolic Ras protein relays the signal through the activation of other components of the MAPK pathway, including the kinases Raf, MAPK kinase (MEK) and extracellular-signal regulated kinase (ERK). Ras and Raf are important molecular signal transducers, and MEK performs the intermediate signaling by phosphorylatying and activating ERK1 and ERK2, the effector molecules in this signaling process.17 The process of tumor angiogenesis, which plays an important role in the development of HCC, also increases the number of receptors required for MAPK cascade signaling, thereby helping to regulate the proliferation, differentiation and survival of tumor cells.18 Other signaling pathways involved in the development of HCC include the pathways known as PI3K/ Akt/mTOR and the Wnt/β-catenin.19 All this knowledge has been used in the development of treatments for HCC that target specific molecular alterations relevant to the pathogenesis of this disease.
Initial Evaluation of Patients With HCCThe diagnosis of HCC is typically made by radiological liver imaging in combination with the evaluation of serum alpha-fetoprotein, a tumor marker that is elevated in 60 to 70% of patients with HCC.20 Treatment for this disease depends on the tumor stage, which can be classified by the tumor-node-metastasis (TNM) system according to the size of the primary tumor (T), the extent of its spread to regional lymph nodes (N), and presence of distant metastasis (M). However, the TNM system evaluates the morphological characteristics of the tumor, but does not take into account its impact on liver function. Thus, the use of a functional system, such as the Child-Pugh classification, is often necessary. The Child-Pugh classification takes into account the most significant factors in the patient with chronic hepatic disease and classifies them into three stages (A, B and C) with progressive degree of complications of cirrhosis.
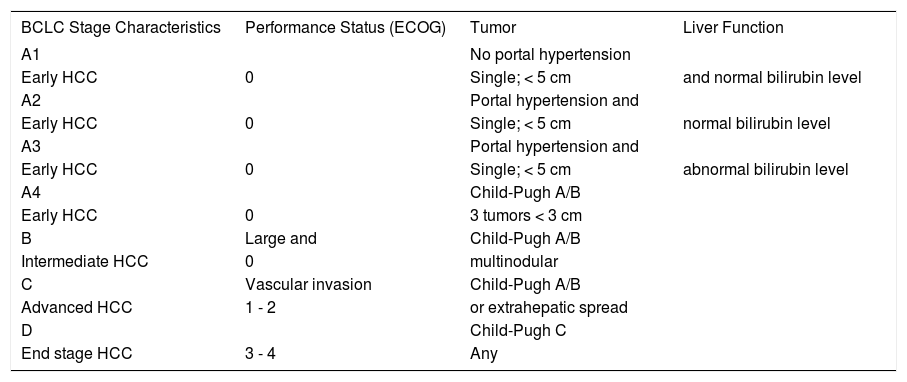
More recently, systems specially developed for HCC have been used with increasing frequency. The Okuda classification 21 was the first to consider the liver function and the levels of albumin and bilirubin, as well as the tumor size, allowing the projection of an expected survival according to these characteristics. Another classification that takes into account characteristics of HCC is known as CLIP (Cancer of the Liver Italian Program);22 by incorporating the Child-Pugh classification, the CLIP system also allows for estimation of the survival expectancy of patients with HCC.23 The Classification of Barcelona (BCLC, Barcelona Clinic Liver Cancer), described in Table 1, is widely used nowadays and was created to stratify patients into treatment groups according to the extent of disease, as well as to predict patient outcome.24
Barcelona Classification (BCLC, Barcelona Clinic Liver Cancer)
| BCLC Stage Characteristics | Performance Status (ECOG) | Tumor | Liver Function |
|---|---|---|---|
| A1 | No portal hypertension | ||
| Early HCC | 0 | Single; < 5 cm | and normal bilirubin level |
| A2 | Portal hypertension and | ||
| Early HCC | 0 | Single; < 5 cm | normal bilirubin level |
| A3 | Portal hypertension and | ||
| Early HCC | 0 | Single; < 5 cm | abnormal bilirubin level |
| A4 | Child-Pugh A/B | ||
| Early HCC | 0 | 3 tumors < 3 cm | |
| B | Large and | Child-Pugh A/B | |
| Intermediate HCC | 0 | multinodular | |
| C | Vascular invasion | Child-Pugh A/B | |
| Advanced HCC | 1 - 2 | or extrahepatic spread | |
| D | Child-Pugh C | ||
| End stage HCC | 3 - 4 | Any |
ECOG, Eastern Cooperative Oncology Group.
For years, the only potentially curative treatments for selected patients with small HCCs were partial hepatectomy and liver transplantation. Currently, for patients who are not surgical candidates due to the severity of liver disease or advanced tumor stage, there are options such as local ablative therapy (radiofrequency, microwave coagulation therapy, or percutaneous ethanol injection), transarterial techniques (transarterial embolization, transarterial chemotherapy, transarterial chemoembolization, and arterial radioembolization), and some forms of extracorporeal energy therapy.22
In the last two decades, due to a better understanding of liver segmental anatomy, there has been an increase in the use of resection as a treatment modality for HCC. This has been associated with 5-year overall survival rates ranging from 30% to 60%, with the current rate of operative mortality being less than 3%. Despite these satisfactory results, only 10% to 30% of HCC cases are eligible to surgical resection with curative intent at the time of tumor diagnosis.22 HCC recurrence is observed in 50 to 80% of patients at 5 years after completion of the resection, with most relapses occurring within 2 years.25 In this same period, the concept of liver transplantation as the treatment for HCC has also evolved and is theoretically better than partial hepatectomy, but the criteria for its use is still under debate.22
In advanced disease, not eligible to surgical resection or other forms of treatment with curative potential, treatment options were very limited until recently. Systemic treatment has been suggested as potentially beneficial to some of these patients, especially with the use of tamoxifen and doxorubicin. However, in a meta-analysis by Llovet and Bruix, published in 2003,26 it was noted that tamoxifen does not provide a significant anti-tumor effect or benefit in survival for patients with advanced HCC. The use of systemic chemotherapy has also been discouraged for the treatment of these patients. Studies in patients treated with doxorubicin showed no evidence of improvement in survival rates, and only about 10% of patients responded to this treatment.20
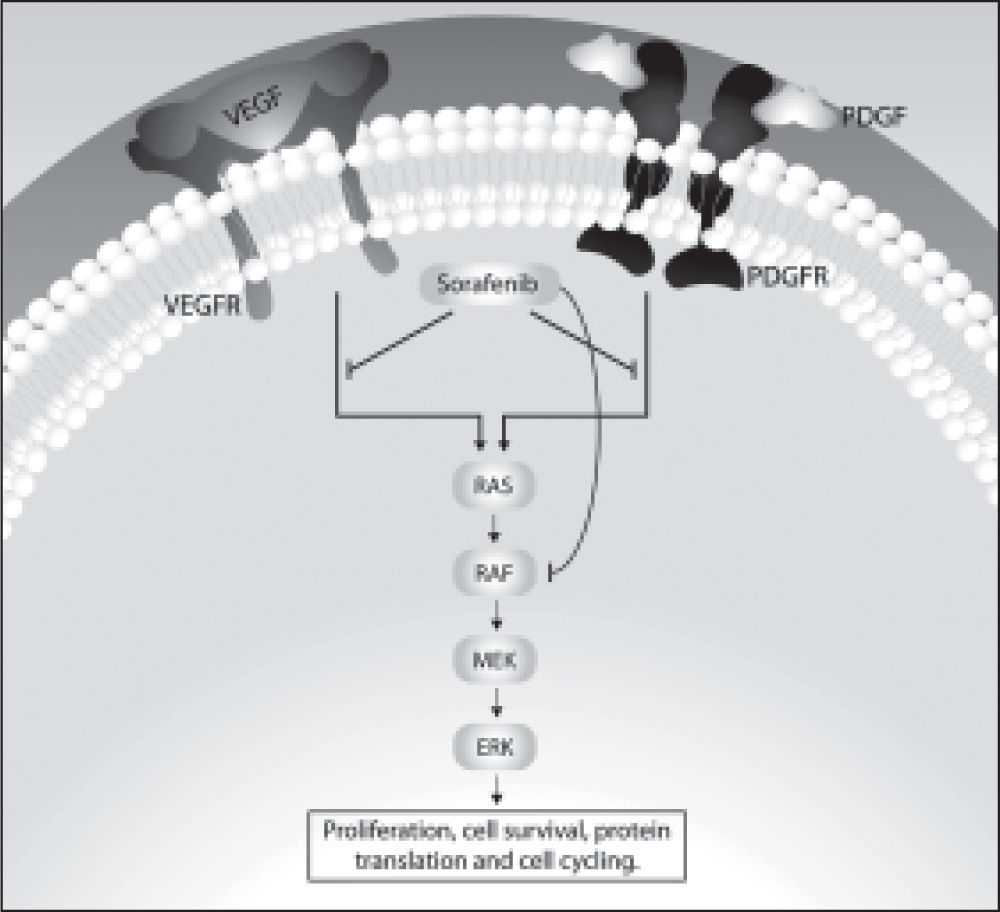
Molecular Targeted Drugs for Advanced HccTreatment options for patients with advanced HCC are extremely limited, but the identification of signaling pathways, as mentioned earlier, and the recognition of the role of these pathways in the pathogenesis of the disease resulted in the development of drugs directed at specific therapeutic targets.19,20 One such drug is sorafenib, also known as BAY 439006. Sorafenib interferes with the MAPK signaling pathway by being able to inhibit some membrane receptors with intrinsic tyrosine kinase activity and also the serine/threonine kinase Raf.26 Among the tyrosine kinases inhibited by sorafenib are the receptors for VEGF (VEGFR-1, VEGFR-2 and VEGFR-3), the beta receptor for PDGF (PDGFR-beta), the c-KIT receptor, the receptor for the macrophage-colony stimulating factor (FLT3), and the RET receptor. Sorafenib is also responsible for the inhibition of both wild-type and mutated forms of the intracellular Raf serine/threonine kinase isoforms, including Raf-1 (or C-Raf) and B-Raf. These kinases are involved in cell proliferation and tumor angiogenesis.27,28Figure 1 illustrates these cellular events and the potential role of sorafenib in the pathogenesis of HCC.
The anti-tumor activity of sorafenib has been shown to be dose-dependent when evaluated in a murine xenograft model in the human hepatoma cell line PLC/PRF/5. In the same study, a 49% inhibition in tumor growth when using a daily dose of 10 mg/kg was observed, and a 30 mg/kg dose completely inhibited tumor growth for 21 days. In this model, these same doses of sorafenib administered for 5 days also inhibited tumor angiogenesis and signaling through Raf/MEK/ERK pathway, and the drug was able to induce apoptosis.29
In humans, sorafenib was evaluated in several phase I studies, which showed that the drug is well tolerated when administered orally and has activity in some human tumors.30-33 In a phase II study that included 137 patients with inoperable HCC without prior systemic treatment and classified as Child-Pugh A or B, sorafenib was administrated in continuous daily oral dose of 400 mg every 12 hours in cycles of 4 weeks. In this study, a median overall survival of 9.2 months and a median time to tumor progression of 4.2 months were noted.34
The efficacy of sorafenib as monotherapy in patients with advanced HCC was evaluated in two phase III, multicenter and double-blind trials: the SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomized Protocol)35 study and a study conducted in the Asia-Pacific region. 36 In the SHARP study, 602 patients were randomized to receive oral sorafenib (400 mg twice daily) or placebo until both radiological and symptomatic progression. This study, conducted from March 2005 to April 2006, demonstrated that the use of sorafenib twice daily prolonged the overall survival of patients with HCC. There was no difference in the median time to symptomatic progression between the treatment groups (sorafenib group: 4.1 months; placebo group: 4.9 months; hazard ratio [HR]: 1.08, 95% confidence interval [CI]: 0.88 – 1.31), but there was a delay in radiological progression in patients treated with sorafenib, compared with those receiving placebo (sorafenib group: 5.5 months; placebo group: 2.8 months, HR: 0.58, 95% CI: 0.45 – 0.74; P < 0.001). Thus, sorafenib was the first molecular targeted agent to demonstrate a statistically significant improvement in overall survival rates in patients with advanced HCC.
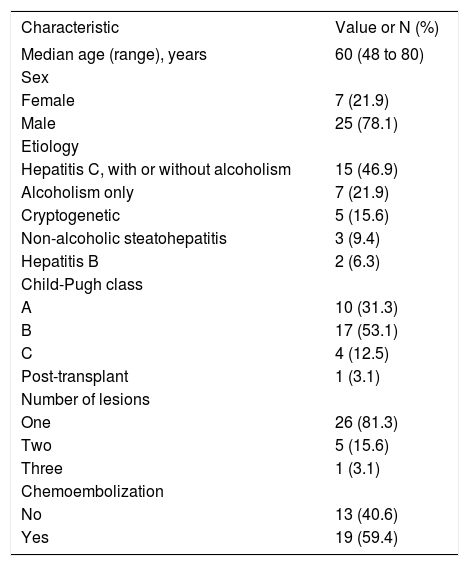
Results that were similar to those of the SHARP study were observed in Brazil.37,38 In this retrospective study, 32 patients were treated with sorafenib and showed a median overall survival of 11 months, after a median time on treatment of 9.4 months. The demographic and clinical profile of patients included in this study is shown in Table 2. It should be noted that around half of the patients were in Child-Pugh class B, while the SHARP study had virtually limited inclusion to patients in class A. Furthermore, it is important to note that there is still limited experience with the use of sorafenib in class B patients. A subgroup analysis of the phase II study mentioned above suggests that in these patients sorafenib is well tolerated and demonstrates antitumor activity. Besides, there are no pharmacokinetic differences between patients in classes A and B.34
Demographic and Clinical profile of advanced HCC patients evaluated in the study conducted in Brazil.37
| Characteristic | Value or N (%) |
|---|---|
| Median age (range), years | 60 (48 to 80) |
| Sex | |
| Female | 7 (21.9) |
| Male | 25 (78.1) |
| Etiology | |
| Hepatitis C, with or without alcoholism | 15 (46.9) |
| Alcoholism only | 7 (21.9) |
| Cryptogenetic | 5 (15.6) |
| Non-alcoholic steatohepatitis | 3 (9.4) |
| Hepatitis B | 2 (6.3) |
| Child-Pugh class | |
| A | 10 (31.3) |
| B | 17 (53.1) |
| C | 4 (12.5) |
| Post-transplant | 1 (3.1) |
| Number of lesions | |
| One | 26 (81.3) |
| Two | 5 (15.6) |
| Three | 1 (3.1) |
| Chemoembolization | |
| No | 13 (40.6) |
| Yes | 19 (59.4) |
Patients evaluated in the SHARP study had hepatitis C (29%), alcoholism (26%) and hepatitis B (19%)35 as the main causes of HCC, with similar distributions observed in the study conducted in Brazil (hepatitis C, 47%; alcoholism, 22%, hepatitis B, 6%).(37) The incidence of adverse events related to treatment with sorafenib in the SHARP study was 80% in patients who received the drug and 52% in the placebo group. Adverse events reported by patients receiving sorafenib were predominantly gastrointestinal, constitutional or dermatologic. Diarrhea, weight loss, hand-foot syndrome, alopecia and anorexia were more frequent in the group treated with sorafenib, compared with the placebo group. Adverse events that often led to dose reduction of sorafenib were diarrhea (8%), hand-foot syndrome (5%) and rash/desquamation (3%); only 34 of the patients (11%) treated with sorafenib had to be permanently discontinued from the study.(35) In general terms, adverse events associated with sorafenib are easily managed, which improves patients' adherence to treatment.
In the phase III study conducted in the Asia-Pacific region, 226 patients were randomized to receive either oral sorafenib (400 mg twice daily) or placebo.36 The results of this study corroborated the ones from the SHARP study, with a median overall survival of 6.5 months in the group treated with sorafenib and 4.2 months in the placebo group. The Asian patients had more advanced disease than those included in the SHARP study, which may have been responsible for the lower efficacy results in both treatment groups (placebo and sorafenib) in the Asia-Pacific study. It is important to note that the gain in median survival in the SHARP and Asia-Pacific studies translate into a reduction of the risk of death of around 30%, since these studies found hazard ratios of 0.69 and 0.68, respectively.
Prospects for the FutureA better understanding of the primary molecular defects that drive tumor cell proliferation has been critical to the development of more effective and less toxic therapies. These molecular defects represent therapeutic targets susceptible to pharmacological intervention, with prospects for the development of several new drugs capable of modifying the natural history of HCC.12,28 Among the current most promising drugs are bevacizumab, a monoclonal antibody against VEGF,37 EGFR inhibitors (erlotinib, cetuximab and gefitinib, among others) and drugs from other classes.39,40
ConclusionAlthough treatments administered in the early stages of HCC, such as surgical resection, local ablation and liver transplantation have curative potential, most patients with HCC present at advanced stages at diagnosis. Sorafenib is an oral inhibitor of multiple important kinases in the pathogenesis of HCC, with potent anti-angiogenic and antiproliferative effects. Sorafenib has demonstrated survival benefits in patients with advanced HCC, thus representing a new standard reference for systemic treatment in these cases.
AcknowledgmentWe thank the editorial support provided by Daniela Mirandola Ferreira, PhD and her team at Dendrix Research, Ltd. We also thank Bayer Schering Pharma Brazil for financial support.