Introduction. Autoimmune hepatitis (AIH) and overlap-syndrome (OS) are autoimmune liver diseases of unknown etiology. Although HLA-DR3/DR4 plays a susceptibility role in AIH but there is limited information in regard to OS.
Objective. Determine the genetic expression of HLA-DR among patients with AIH versus OS in order to establish susceptibility alleles in comparison to healthy controls (HC).
Methods. 26 patients with AIH and 15 patients with OS were studied. Ninety-nine healthy historical controls without autoimmunity were evaluated. Patients with AIH and OS were selected based on the international group for the study of AIH criteria and the Chazouilleres criteria for OS. Patients had at least one liver biopsy. Characterization of HLA-DR was extracted from peripheral blood leukocytes. Alleles were obtained for AIH, OS and HC and comparisons were made between groups.
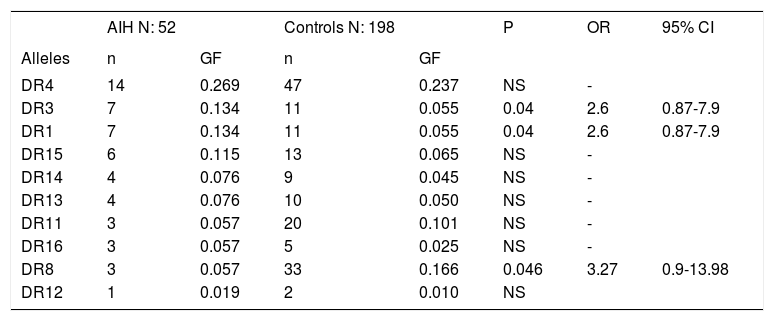
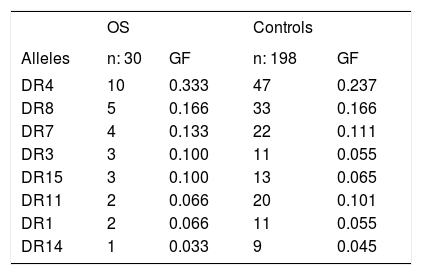
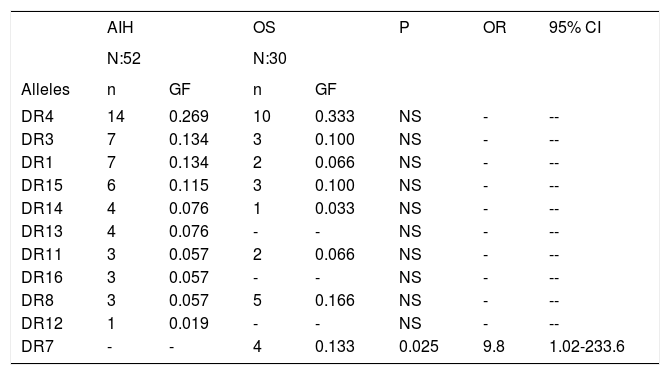
Results. There was a significant increase in HLA-DR3 and DR1 in AIH compared with the HC group (p = 0.04, OR 2.6, 0.87-7.9, 95% CI). In the AIH group there was a decreased frequency in allele HLA-DR8 when compared with HC (p = 0.04, OR 3.2). There were no statistical differences between the genetic frequencies in the OS group compared with HC. However, HLA-DR7 was able to distinguish between OS patients from those with AIH (p = 0.02, OR 9.8, 1.02-233.6, 95% CI).
Conclusions. HLA-DR1/DR3 is increased in AIH, but contrary to data reported in AIH, HLA-DR7 frequency is increased in OS, suggesting increased susceptibility which distinguishes patients with AIH from those with OS.
The overlap-syndrome (OS) of autoimmune hepatitis and primary biliary cirrhosis (AIH-PBC) is a primary autoimmune liver disease. Although it shares the clinical, biochemical, serological and histological features of patients with type 1 autoimmune hepatitis and primary biliary cirrhosis, it seems to have a distinctive clinical course that requires different therapeutic interventions.
Susceptibility genes in patients with type 1 AIH are associated with the mayor histocompatibility complex (MHC), more specifically with the locus HLA-DR (HLA-DR3/DR4) in different populations.12
There are few reports related to susceptibility genes in OS and some authors postulate that it could be linked to a form of PBC due to the characteristic histological damage in biliary ducts and positivity of antimitochondrial antibodies. On the other hand, OS patients share alleles HLA-B8, DR-3 and DR-4 which are more frequently prevalent in patients with type 1 AIH and not with PBC.3
A study in Mexican patients with AIH showed that HLA-DR4 is associated with an increased risk for the development of other autoimmune diseases like systemic lupus erythematosus when compared with controls. However there was no comparison with OS patients.4 Previous reports from mestizo Mexican patients have found an increased allelic frequency of HLA-DR 4 and DR1 in those patients with AIH, PBC and OS. They found that HLA-DR8 is not present in any of this autoimmune diseases compared con healthy historical controls, postulating that this allele could have a protecting role.5 There are no reports comparing the genetic association of HLA-DR locus in patients with AIH and those with OS in order to establish their respective genetic frequencies compared with controls without autoimmune liver disease.
The aim of this study was to establish the genetic frequencies in patients with AIH and OS(AIH-PBC) in a selected group of patients and determine alleles at risk that could confer a protective effect of this entity.
Material and MethodsPatientsPatients were prospectively enrolled from April 2007 to October 2007. There were 26 patients with AIH and 15 patients with OS.6 Patients with OS were diagnosed based on the criteria established by Chazouillères, et al.:7 Features of PBC were:
- •
Serum alkaline phosphatase (ALP) more than twice the upper limit of normal value and/or gammaglutamyltransferase (GGT) five or more times the upper limit of normal value.
- •
Positivity for AMA.
- •
Evidence histological bile ducts damage.
Features of AIH were the following:
- •
Aminotransferase (ALT) elevation at least five times the upper limit of normal value.
- •
Levels of IgG immunoglobulin at least twice the upper limit of normal value and/or positivity to smooth muscle antibodies.
- •
Liver biopsy showing interface hepatitis with moderate to severe periportal lymphocyte infiltration.
Diagnosis was considered when there was presence of 2/3 criteria. The diagnosis of AIH was based on criteria of the International Autoimmune Hepatitis Group (IAIHG). Patients were selected if they had 15 points or more pretreatment and / or 17 points or more after treatment. We excluded patients with hepatitis B, C and/or with any alcohol consumption. There was no exposure to hepatotoxic drugs. Informed consent was obtained and approved by the ethics committee from our hospital. The study was approved by the Institutional Review Board of our Institution.
SerologyAll AIH patients were positive for anti-nuclear antibodies by indirect immunofluorescence and negative for antimitocondrial antibodies M2 fraction. Patients with OS, were positive for antimitocondrial M2 fraction through ELISA method and were positive for antinuclear antibodies and/or positivity for anti-smooth muscle antibodies through immunofluorescence. All patients were tested and were negative for anti-LKMl autoantibodies.
HistologyLiver biopsy was performed for diagnostic purposes in all AIH and OS patients before therapy. The liver biopsies were evaluated by different pathologists in different periods of time looking for bile ducts damage, presence of interface hepatitis, lymphocyte infiltration and piecemeal necrosis.
DNA/HLA determinationLeukocytes were extracted from peripheral blood samples and subsequently separated in order to obtain DNA by protein-kinase extraction with phenolchloroform and isopropanol according to the protocol to the Twelfth international consensus of histocompatibility.8 DNA amplification was carried by PCR in the locus of HLA-DR.
The PCR products were denaturized and placed in nylon membranes, and then UV irradiation was applied. The membranes were laundered with specific sequence primers and then incubated with antibody antiDIG by 60 minutes. After removing excess, hybridization was performed through chemoluminesence. Primers specific sequences (PCR-PSS) were magnified to confirm the HLA-DR loci.
ControlsWe included one-hundred ninety-eight adult (age > 18 yr) historical controls without any other autoimmune disease, included their first degree relatives.
Statistical analysisStatistical analysis was performed to detect differences in genetic frequencies for AIH and OS as well as for the control group. We compared the genetic frequencies among patients with AIH and controls and OS vs. controls by Χ2 test. In those alleles in which a significant difference was detected, odds ratio were obtained to assess the strength of the association. We compared patients with AIH versus OS through the two-tail Fisher exact test when the expected number of subjects was less than 5. A value of p ≦ 0.05 was considered statistically significant.
ResultsTables 1 and 2 show the genetic frequencies of patients with AIH vs. controls and those with OS, respectively. In the case of alleles HLA-DR3 and DR1, there was a statistically significant increase in AIH compared with the control group [p = 0.04 and OR 2.6 (0.81-1.9, 95% CI)].On the other hand, in the group of AIH there was a statistically significant decrease frequency in allele HLA-DR8 when compared with controls p = 0.04 with an OR 3.2 (0.9-13.98, 95% CI). The analysis of the genetic frequency of subjects with OS showed that the alleles HLA-DR do not differ from those of the controls. Finally, when comparison was made between the genetic frequencies of AIH patients against those with OS (Table 3), HLA-DR7 was able to distinguish patients with OS from those with AIH, showing a significant statistical difference with a value of p = 0.02 and OR of 9.8 (1.02-233.6, 95% CI).
Gene frequencies of the alleles in patients with AIH compared with controls
| AIH N: 52 | Controls N: 198 | P | OR | 95% CI | |||
|---|---|---|---|---|---|---|---|
| Alleles | n | GF | n | GF | |||
| DR4 | 14 | 0.269 | 47 | 0.237 | NS | - | |
| DR3 | 7 | 0.134 | 11 | 0.055 | 0.04 | 2.6 | 0.87-7.9 |
| DR1 | 7 | 0.134 | 11 | 0.055 | 0.04 | 2.6 | 0.87-7.9 |
| DR15 | 6 | 0.115 | 13 | 0.065 | NS | - | |
| DR14 | 4 | 0.076 | 9 | 0.045 | NS | - | |
| DR13 | 4 | 0.076 | 10 | 0.050 | NS | - | |
| DR11 | 3 | 0.057 | 20 | 0.101 | NS | - | |
| DR16 | 3 | 0.057 | 5 | 0.025 | NS | - | |
| DR8 | 3 | 0.057 | 33 | 0.166 | 0.046 | 3.27 | 0.9-13.98 |
| DR12 | 1 | 0.019 | 2 | 0.010 | NS |
AIH: Autoimmune hepatitis. GF: Genetic frequency.
Genetic frequencies of the alleles in patients with OS compared with controls.
| OS | Controls | |||
|---|---|---|---|---|
| Alleles | n: 30 | GF | n: 198 | GF |
| DR4 | 10 | 0.333 | 47 | 0.237 |
| DR8 | 5 | 0.166 | 33 | 0.166 |
| DR7 | 4 | 0.133 | 22 | 0.111 |
| DR3 | 3 | 0.100 | 11 | 0.055 |
| DR15 | 3 | 0.100 | 13 | 0.065 |
| DR11 | 2 | 0.066 | 20 | 0.101 |
| DR1 | 2 | 0.066 | 11 | 0.055 |
| DR14 | 1 | 0.033 | 9 | 0.045 |
OS: Overlap syndrome. GF: Genetic frequency.
Genetic frequencies of the alleles in patients with AIH compared with patients with OS.
| AIH | OS | P | OR | 95% CI | |||
|---|---|---|---|---|---|---|---|
| N:52 | N:30 | ||||||
| Alleles | n | GF | n | GF | |||
| DR4 | 14 | 0.269 | 10 | 0.333 | NS | - | -- |
| DR3 | 7 | 0.134 | 3 | 0.100 | NS | - | -- |
| DR1 | 7 | 0.134 | 2 | 0.066 | NS | - | -- |
| DR15 | 6 | 0.115 | 3 | 0.100 | NS | - | -- |
| DR14 | 4 | 0.076 | 1 | 0.033 | NS | - | -- |
| DR13 | 4 | 0.076 | - | - | NS | - | -- |
| DR11 | 3 | 0.057 | 2 | 0.066 | NS | - | -- |
| DR16 | 3 | 0.057 | - | - | NS | - | -- |
| DR8 | 3 | 0.057 | 5 | 0.166 | NS | - | -- |
| DR12 | 1 | 0.019 | - | - | NS | - | -- |
| DR7 | - | - | 4 | 0.133 | 0.025 | 9.8 | 1.02-233.6 |
AIH: Autoimmune hepatitis. OS: Overlap syndrome. GF: Genetic frequency.
The results of this study reveal that alleles HLA-DR3 and DR1 are related with AIH and that HLA-DR8 it is protective. There was no influence in any allele class DR in OS but HLA-DR7 was able to distinguish between AIH and OS. Previous studies have shown that the genetic expression leads to different a presentation of the disease in AIH; that is the case of DRB1*0301 in younger patients, showing severe disease comparing to those with DRB1*0401.9-12 There are different genes that modify the phenotypic expression of AIH, which include the presence of TNFA*2 polymorphisms, associated with poor response to treatment with steroids13,14 and the presence of CTLA-4 which is related with extra-hepatic manifestations and TNFRSF6 with progression to cirrhosis.15 In this way, DRB1*0301 appears to be a gene driver and when it is associated with CTLA-4 and TNFA*2 polymorphisms it may show a modification in the expression of the disease.16
It is possible to speculate that drivers of hepatic expression are first determined by HLA-DR alleles; however, it is possible the coexistence of secondary genes not well known inside and outside of the MHC, could modify phenotypic expression.17 The recognition of these alleles and their function could lead to a more in-depth knowledge of the physiopathology of AIH that may lead to important implications in treatment and outcome.
Another interesting concept relates with gene dosing, where the presence of 3-4 alleles that codify lysine in the DRB71 position affects expression of disease.2 It seems that different kinds of expression in AIH are related with the regulation of other genes.18 The same occurs in OS, where shared haplotypes drivers show a difference by the presence of HLA-DR7, which means that this subgroup of patients could have a different disease course, compared to those who share AIH haplotypes. Previous studies have not shown the presence of HLA-DR7 in patients with AIH.41019 The knowledge of the genetic drivers and its associations with genes that modify its course in patients with AIH and OS will lead an accurate genetic diagnosis that could finally impact in treatment and prognosis of these patients.17 Interestingly, as previous reports in mexican mestizo patients, it seems that HLA-DR8 is not present in patients with both autoimmune diseases, suggesting that this allele could have a protecting role in this population.5
A major limitation of this our study is a low sample size and lack of validation in a different ethnic population, nonetheless the low prevalence of both AIH and OS makes it very difficult to recruit a larger number of patients. However, we were able to collect blood samples in a prospective fashion in highly selected patients. Despite the small sample, we demonstrated differences between AIH and OS, and perhaps, a larger sample size probably could improve the results, by detecting more differences between AIH and OS and OS compared to control population avoiding type II error.
Based on genetic frequency, we detected and increase in HLA-DR7 in OS comparing with AIH but not differences were detected making comparison between OS against controls. We hypothesized that there are other genes related with the development of OS and there are perhaps, with the joint of this genes, there is the optimal condition that triggers the disease. As is well know, this disease is multifactorial in nature and there is not a single marker that has been used for its diagnosis.
In conclusion, the results of this study confirm the preponderant role of HLA-DR in the genetic susceptibility for the development of hepatic autoimmunity. We have found an increase in the genetic frequency of the alleles DR3 and DR1 in AIH while HLA-DR7, may be involved in the susceptibility to development of OS and may be a possible marker that could help to distinguish OS from AIH. Finally, DR8 probably protects against hepatic autoimmunity.
Conflict of InterestThe authors have no conflict of interest to report.