Alcohol is the leading cause of preventable liver morbidity and mortality worldwide, as it is also the most frequent cause of advanced liver disease. Alcohol-associated liver disease (ALD) covers different phenotypes ranging from steatosis to the development of inflammation (steatohepatitis), fibrosis and ultimately, in a proportion of patients, the development of liver cirrhosis and its associated complications. ALD has a complex pathogenesis that includes the interplay of both genetic and environmental factors, yet the precise mechanisms are largely unknown. Alcohol-associated hepatitis (AH) is a severe clinical presentation of ALD, which is characterized by abrupt jaundice and clinical decompensations of liver disease. AH occurs in a percentage of patients with underlying ALD and active alcohol consumption. Currently, there are no approved targeted therapies able to interfere in the pathogenesis of ALD and halt the progression of the disease, therefore alcohol abstinence is the most effective measure to improve prognosis in this patient population. In this regard, alcohol cessation remains the first-line treatment in all stages of alcohol disease. In patients with advanced ALD nonresponding to medical therapy, liver transplantation is the only approach that improves prognosis, and it should be considered in patients with decompensated cirrhosis. In the last years, AH has emerged as a new indication of early liver transplantation in non-responders to medical therapy, with promising results in highly selected patients. In this review, we provide an update on the epidemiology, risk factors, natural history, diagnosis, pathogenesis, and current treatments for ALD, taking into account the importance of assessing and managing alcohol consumption as the etiological factor and the main driver of prognosis in patients with ALD.
Alcohol-associated hepatitis
Alcohol-associated liver disease
Alcohol use disorder
Hepatitis B virus
Hepatocellular carcinoma
Hepatitis C virus
Liver transplantation
Metabolic associated fatty liver disease
Maddrey modified discriminant function
Model for End-Stage Liver Disease
Spontaneous bacterial peritonitis
World Health Organization.
Hazardous alcohol consumption caused in 2018 roughly 3 million deaths globally (5.3 % of all deaths) according to the World Health Organization (WHO) [1]. Alcohol is the seventh leading risk factor for both premature deaths and disabilities worldwide [2]. It has a deleterious effect on an individual's health, being responsible for cardiovascular and gastrointestinal diseases as well as cancer development (liver and non-liver neoplasms) [2,3].
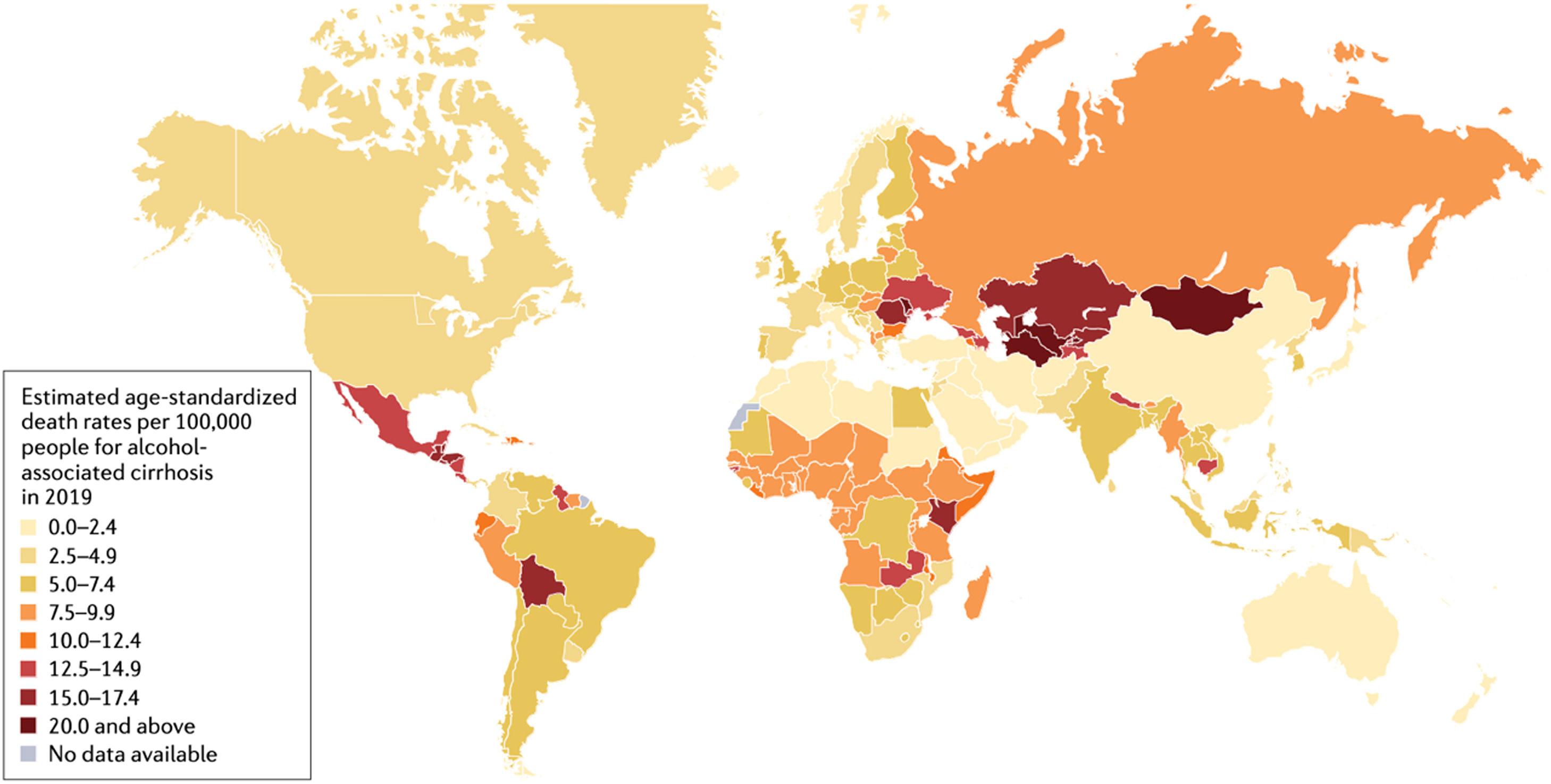
There are geographical differences regarding alcohol-associated morbidity and mortality; the WHO European Region being the most affected area [4]. Per capita, alcohol consumption is highest in Europe, followed closely by the countries of the Region of the Americas (Fig. 1). Not surprisingly, the region of the Americas is second to the European countries in terms of alcohol-attributable deaths and disability-adjusted life-years attributable to alcohol worldwide [5,6]. It should be pointed out that the impact of alcohol in Latin America is enhanced by social inequities and health determinants such as deficiencies in healthcare provision [7].
Worldwide prevalence of death rates related with alcohol-associated cirrhosis (with permission from Huang D, et al. [136].
The Diagnostic and Statistical Manual of Mental Disorders (DSM-V) defines alcohol use disorder (AUD) as the chronic inability to stop alcohol use despite adverse social, occupational and health consequences [8,9]. Alcohol-associated liver disease (ALD) encompasses a broad spectrum of liver injuries that can eventually lead to cirrhosis, being the most frequent cause of cirrhosis worldwide [10]. Both ALD and AUD have a significant impact on countries’ economies. First, there are labor repercussions due to absenteeism, sick leave, early retirement, and premature mortality [11,12], since liver diseases usually occur in working age individuals. Second, healthcare expenditures derived from the care of AUD and ALD are increased. It has been estimated that ALD is responsible for more than half of the inpatient charges for liver disease [13,14].
Alcohol consumption also has detrimental effects on chronic liver disease due to other etiologies; therefore, abstinence should be the mainstay for all patients with liver disease since no safe threshold exists.
1.2Patterns of consumptionThe frequency and amount of alcohol use are the two main driving factors of disease progression. Across all age groups the prevalence of alcohol consumption is greater in men than in women [4]. The amount above which alcohol may start increasing morbidity and mortality has been explored in epidemiological studies. Although the evidence on the effect of low amounts of alcohol consumption as a risk factor for mortality in some age ranges is heterogeneous, possibly due to the different data sources used in the studies that recently explored this association, it seems clear that amounts of alcohol above 1–2 units per day are associated with an increased overall mortality in general population in a dose-dependent manner [15,16]. Regarding the risk of liver disease, the current evidence suggests that alcohol consumption has a direct proportional relationship with the risk of liver disease, with similar consequences described in women with lower amounts of alcohol consumption compared to men [17]. Proposed explanations include lesser body water content and the lower amount of gastric alcohol dehydrogenase activity in women [18,19].
Harmful alcohol consumption is defined as a daily intake of more than 2 standard drinks in men and 1 standard drink in women, or the presence of binge drinking. According to the WHO [20], binge drinking is defined as consumption more than 5 standard drinks for men and more than 4 in for women in a 2-hour period [21]. Importantly, while binge drinking probably does not cause liver disease by itself, it accelerates fibrosis progression and development of cirrhosis in people with obesity and metabolic syndrome [22].
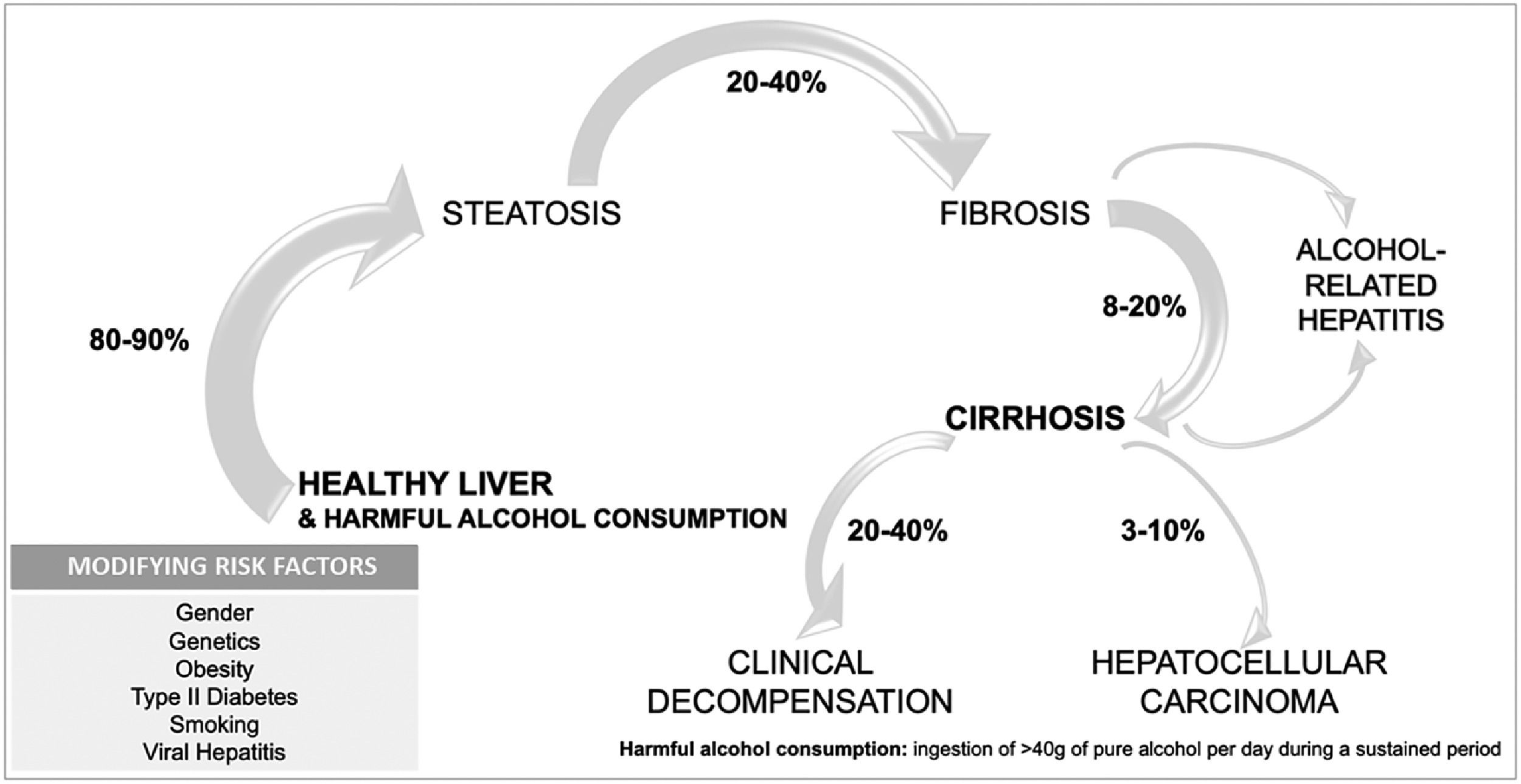
1.3Natural history of alcohol-associated liver diseaseALD includes several phenotypes that range from asymptomatic disease to advanced forms with a high mortality rate. ALD can advance from steatosis to steatohepatitis and fibrosis, with the appearance of cirrhosis and its complications, and in some cases hepatocellular carcinoma [23] (Fig. 2). Different factors interplay in the progression of the disease, since 90 % of heavy drinkers will develop simple steatosis, while 10–20 % will progress to cirrhosis [24,25]. Cirrhosis can develop after 10 years of harmful drinking, in contrast to other slower etiologies [23]. To date, there are no well-validated tools to predict who will develop advanced liver disease secondary to alcohol consumption.
Alcohol-associated hepatitis (AH) is an acute deterioration that occurs in people with underlying ALD, and mostly in the presence of cirrhosis. AH encompasses steatohepatitis, pericellular fibrosis, hepatocellular injury and bilurrubinostasis. The prevalence is not well known, because of disparities in AH diagnosis, but it is believed to occur in 10–35 % of patients with advanced fibrosis and heavy alcohol intake [26].
The risk of developing cirrhosis in the setting of AUD is related to host susceptibility, including genetic and environmental factors [27]. Genetic variations associated with an increased risk of cirrhosis have been reported. Mutations in patatin-like phospholipase domain–containing 3 (PNLAP3), transmembrane 6 superfamily member 2 (TM6SF2), and membrane-bound O-acyltransferase domain-containing protein 7 (MBOAT7) predispose to ALD [23,28]. On the other hand, polymorphisms in the gene encoding hydroxysteroid 17-beta dehydrogenase 13 (HSD17B13) have shown a protective role. There is also gender-based differences in the risk of ALD, being consistently women at higher risk of progression to liver disease with similar amount of alcohol consumption.
A special mention should be made when assessing the interaction between alcohol consumption and metabolic risk factors as cofactors for liver disease. These two agents are currently the two most frequent and relevant causes of liver disease in Europe and the Americas [29]. It seems that the interaction between both factors is bi-directional: alcohol impacts the progression of MAFLD cirrhosis just as obesity influences the natural history of ALD [30,31]. In the case of ALD, people with AUD suffering from overweight or obesity have a higher frequency and risk of advanced forms of ALD [32]. Coexistence of another liver diseases, such as HBV, HCV, or hemochromatosis, also increases the chances of developing cirrhosis. It has been estimated that there is a nine-fold risk of liver injury in patients with hemochromatosis and alcohol misuse [33]. Smoking has a synergistic effect on fibrosis progression and in the appearance of neoplasms [34]. Lastly, combining alcohol intake with known hepatotoxic drugs such as paracetamol, potentiate liver disease [23].
2Invasive and non-invasive diagnosis2.1Diagnosis of alcohol use disorderSince AUD is one of the leading causes of morbidity and mortality worldwide, it is crucial to detect it in an early phase [35]. There are several tools available for the diagnosis of AUD and ALD, however, no single one is ideal as screening test, so the combination of questionnaires and biomarkers is recommended [36].
Validated questionnaires: AUD is categorized by the DMS-V criteria in different severity grades, according to the number of symptoms present in each case. However, alcohol consumption is usually underreported [37], probably because of fear of reprisal on candidacy for liver transplantation (LT) [38,39] and social stigma [40,41]. The Alcohol Use Disorder Identification Test (AUDIT score) has been developed to diagnose alcohol abuse through ten questions (AUDIT score > 8) with 73 % sensitivity and 91 % specificity, and alcohol dependence (AUDIT score > 20) with 85 % sensitivity and 89 % specificity [42]. A concise version, (AUDIT-C) consists of three questions with the same reliability [43]. Other recognized tool is the CAGE questionnaire (Cut down, Annoyed, Guilty, Eye opener), a four questions test found in a metanalysis to have a 71 % sensitivity and 90 % specificity for the diagnosis of AUD when getting more than two positive answers [44]. It is more sensitive to detect alcohol abuse, but less useful for non-severe AUD [45].
Biochemical biomarkers: Routine tests such as AST/ALT ratio, GGT and MCV are handy and inexpensive. They can raise suspicion of AUD but have low sensitivity and specificity [46]. Specifically, elevation of GGT is associated with hepatic steatosis, which can be present in the next 3–7 days after heavy drinking. The AST/ALT ratio is usually greater than 1 in patients with ALD and greater than 2 in patients with AH, because of reduced hepatic ALT activity, alcohol-induced depletion of hepatic pyridoxal 50-phosphate and increased hepatic mitochondrial AST [47].
Alcohol biomarkers: Direct measurement of ethanol in blood or urine could be the gold standard for alcohol detection, but rapid ethanol clearance from the bodily fluids negates its utility for monitoring alcohol relapse. Testing indirect alcohol biomarkers, such as ethylglucorinide (EtG), fatty acid ethyl esters (FAEE) and phosphatidylethanol (PEth) generated by ethanol metabolism have emerged as the main tools for abstinence assurance [48]. EtG is the most used and can detect alcohol intake up to 2 days post-consumption in blood and 3 days in urine. On the other hand, PETh can be detected in blood up to 28 days after alcohol consumption, it correlates with the amount of alcohol ingested and has been reported to have a sensitivity of 90–99 % and a specificity of 100 % for alcohol detection [49].
2.2Diagnosis of alcohol associated-liver diseaseThe diagnosis of ALD relies on a history of excessive alcohol intake, altered laboratories congruent with alcohol use, indirect ethanol detection and the exclusion of other liver diseases.
Imaging assessment: Imaging methods such as abdominal ultrasound, computed tomography, or magnetic resonance; are non-specific for the study of ALD. Its utility relies on characterizing liver morphology to support cirrhosis diagnosis, assess portal hypertension signs and to exclude hepatocellular carcinoma, biliary obstruction, or vascular thrombosis as possible causes of deterioration of liver function or clinical decompensation. Of all the image techniques, abdominal ultrasound is, due to its availability and its accuracy to detect signs of advanced chronic liver disease, the most widely used image technique for the diagnosis of all causes of chronic liver disease [50].
Transient elastography (TE): it is widely validated in the diagnosis of alcohol associated fibrosis and it is a useful tool in the diagnosis of subclinical ALD [51,52]. In the last years there have been different initiatives to develop screening strategies for early detection of liver disease in subjects with high-risk alcohol consumption, showing a high performance of TE to identify subjects with liver fibrosis in this population [53]. However, there are several cofounders that need to be considered, such as the hypertransaminasemia related with active alcohol intake that may alter the results, cholestasis, recent alcohol intake, and the presence of AH [54]. The cutoff values to identify advanced fibrosis in ALD are higher than in other etiologies, stated that a TE > 12–15 kPa is diagnostic of advanced fibrosis once the cofounders are excluded. However, more research needs to be done to establish more accurate cutoff values for subclinical ALD without advanced fibrosis, since the METAVIR scale was first designed for virus-related fibrosis evaluation, and it may underestimate the grade of fibrosis in ALD [55].
Liver biopsy histological assessment: it remains the most definitive test in evaluating ALD, although it is an invasive and costly procedure. Moreover, the findings are subjected to sampling error and interobserver variability. In the initial stages, the typical finding is centrilobular macrovesicular steatosis.
Specifically for AH, staging scores have been designed to assess the prognosis. The Alcoholic Hepatitis Staging Score (AHHS) was validated in histologically proven AH to predict short-term survival. This score is based on the degree of fibrosis, the type of bilirubinostasis, the presence of megamitochondria and the extent of polymorphonuclear infiltration. Briefly, the presence of megamitochondria and extensive polymorphonuclear infiltrate portend a good prognosis; while advanced fibrosis or cirrhosis, as well as canalicular or ductular bilirubinostasis, overshadow the patient's prognosis [56]. Finally, the Study of Alcohol-related LiVer disease in Europe (SALVE) Histopathology Group recently developed a new histological grading system for patients with ALD in its whole clinical spectrum. This newly designed and validated score shows that presence of histological factors of alcoholic hepatitis and advanced cirrhosis predict survival and decompensation in the short-term [57].
3Management of underlying alcohol disorder in patients with alcohol-associated liver disease3.1Psychological therapyALD should be considered as a dual pathology: both a liver and an addiction disease [3]. As with any other substance use disorder, AUD follows a remitting-relapsing course. Abstinence should not be considered a treatment for a restricted period but the starting point of an indefinite and dynamic process [27]. Both non-and pharmacological treatment may help patients maintain abstinence and are not self-excluding. Therefore, clinical focus should be on developing insight into AUD and promoting acquisition of alcohol relapse prevention skills [58].
Brief intervention is a non-confronting counselling strategy that can be done by any healthcare provider in less than 10 min to educate the patient in the harmful effects of alcohol drinking and motivate for a behavior change. Although insufficient in heavy alcohol drinkers, it can be used to reduce alcohol use in patients that do not meet AUD criteria or in combination with pharmacological or other psychotherapeutic interventions [59]. Specific psychosocial and behavioral approaches for AUD include Twelve-Step Facilitation, cognitive and behavioral therapy [27]. Twelve-Step facilitation is the method used in Alcoholics Anonymous meetings. The goal of cognitive and behavioral therapy is to identify alcohol consumption drivers as well as maladaptive habits; to be able to replace alcohol-associated circumstances with alcohol-free ones.
3.2Pharmacological treatmentThere are three treatments approved by the Food and Drug Administration (FDA) for AUD: disulfiram, naltrexone and acamprosate [58]. Disulfiram should not be given to patients with underlying liver disease given its hepatotoxicity. Naltrexone is an opioid receptor antagonist; its goal is to reduce the rewarding and reinforcing effects of alcohol. It should be avoided in patients with opioid use disorder with mu-opioid receptor agonist (i.e., methadone) and in patients with decompensated cirrhosis or profound liver dysfunction [60]. On the other hand, acamprosate reduces alcohol craving symptoms while on a prolonged abstinence and diminishes alcohol intake in AUD patient [61]. Acamprosate is believed to work as an N-methyl-D-aspartate (NMDA) antagonist. It should be used cautiously in patients with renal failure. Additionally, baclofen is a selective gamma-aminobutyric acid (GABA) type B agonist that has shown some benefit in treating AUD [62], although it is not approved by the FDA for AUD treatment. As of today, it is the only drug tested off-label in patients with AUD and with significant liver disease [63].
Overall, the evidence on the safety and efficacy of AUD medications in patients with ALD is very limited and their use in clinical practice for the management of AUD in this population is scarce [64] and further scientific evidence to promote the use of these medications is needed in patients with cirrhosis.
4Management of compensated patientsUntil the onset of decompensation, most liver diseases including ALD have a silent course. Therefore, screening in high-risk individuals is a fundamental tool for early diagnosis, decreasing the risk of preventable liver disease. A global study showed patients with ALD are 14-fold more likely to be evaluated at specialized units presenting with advanced disease in comparison with HCV patients [35] who were referred to liver units in early stages of liver disease. Unlike HCV or HBV, where screening intravenous drug users, imprisoned, or sex workers has become part of daily practice, few individuals with alcohol dependence or misuse are screened for ALD [35].
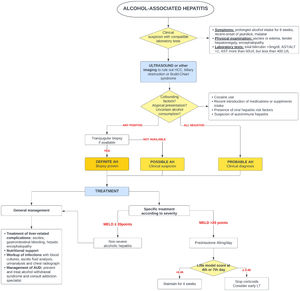
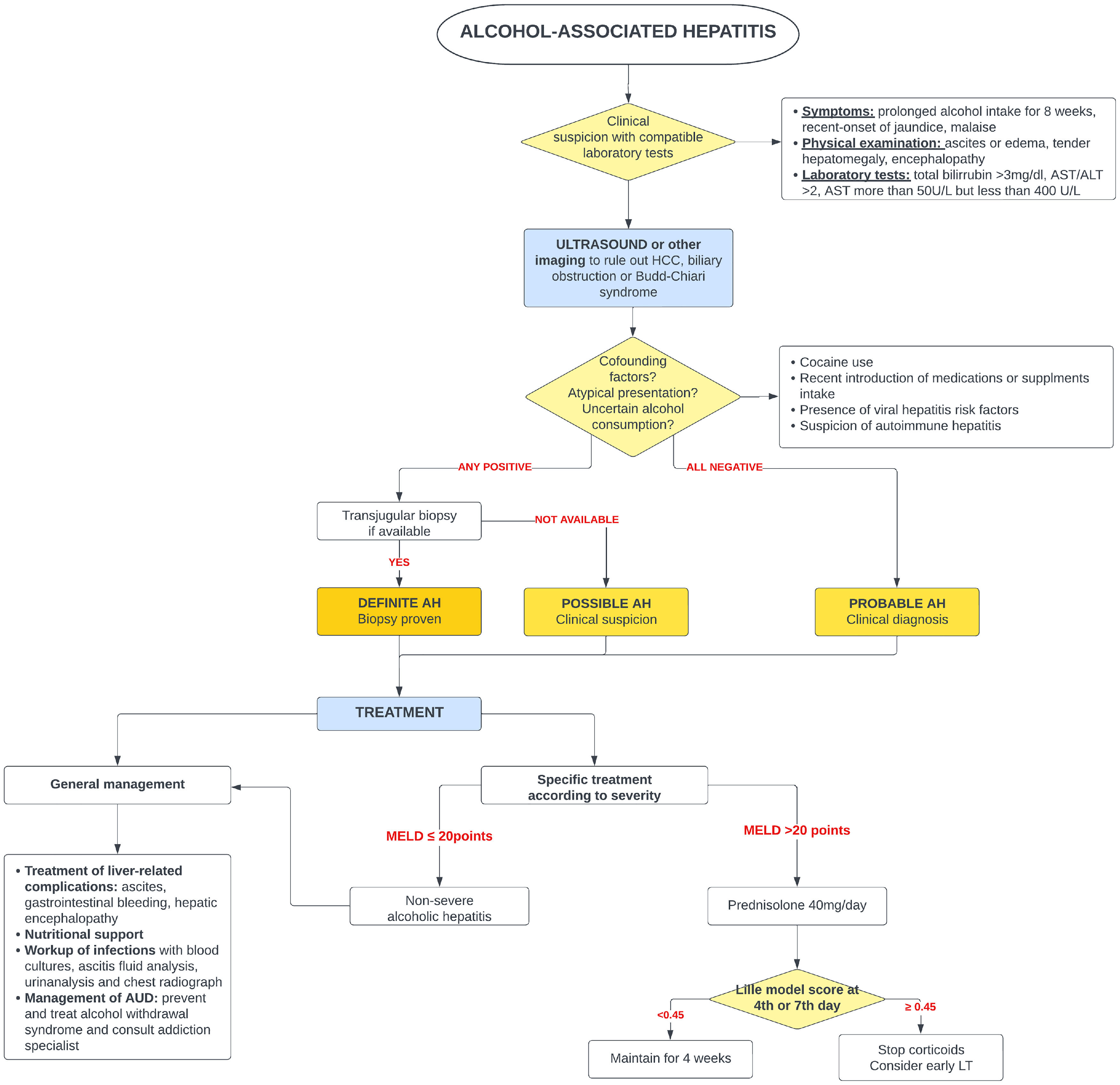
5Alcohol-associated hepatitis5.1Presentation and diagnosisAH presents as an abrupt onset of jaundice in people with active alcohol use and underlying ALD. Average age of presentation is shifting to younger ages and it is becoming more prevalent in women, especially in the United States [65,66]. Severe AH has a grim prognosis with a mortality rate of 20–50 % within 3 months from presentation [67], mostly due to liver-related complications, infections and subsequent multiorgan failure.
The predisposing factors to develop AH among heavy drinking are largely unknown. Both genetic and epigenetic as well as environmental factors probably play a role. The precise interplay between the microbiome, proinflammatory signals and other molecules that condition hepatocyte dedifferentiation and hepatocyte dysfunction remain to be elucidated [68].
AH should be suspected in patients with heavy daily alcohol consumption (>40 g of alcohol for women and >60 g for men) for at least 6 months with less than 60 days of abstinence, who appear with sudden jaundice and liver failure [69]. Besides jaundice, they usually present with anorexia, fever, abdominal pain, and signs of liver decompensation such as ascites, hepatic encephalopathy, or upper gastrointestinal bleeding. Typically, abnormal liver tests include AST between 50 and 300 (no >500 UI), with AST 1.5–2 times greater than ALT and bilirubin equal or greater than 3.0 mg/dl [70].
The diagnosis of probable AH can be made when typical clinical and biochemistry features are present [69]. A transjugular biopsy is indicated when in the presence of confounding factors (e.g., atypical laboratory tests, presence of hepatotoxic drugs, etc.), to provide histological confirmation as well as assess the degree of portal hypertension [46]. Histologically, AH is characterized by macrovesicular steatosis with leucocyte infiltrate, swollen hepatocytes (ballooning degeneration) and Mallory Denk bodies. Other common findings are perisinusoidal and/or perivenular fibrosis (with a “chicken-wire” distribution), megamitochondria, ductular reaction and bilirrubinostasis [71].
It should be kept in mind that not every episode of jaundice in patients with ALD is due to AH. Excluding conditions such as ischemic hepatitis, drug-induced liver injury, viral or autoimmune hepatitis is crucial to adequately treat these conditions and avoid unnecessary corticoid treatment. In addition to ruling out other etiologies, histology is helpful to differentiate AH from alcoholic foamy degeneration (AFD) [72], which is also caused by the chronic damage of alcohol exposure. Differentiating AFD from AH is of paramount importance as AFD improves with abstinence and does not require specific treatment with corticosteroids. Clinical presentation of AFD is similar to AH, however AFD should be suspected when presented with higher aminotransferases and characteristically high triglycerides and cholesterol levels [73,74]. On microscopy, it displays a massive microvesicular steatosis (51) and does not have a polymorph infiltrate.
5.2General managementScreening for infections is one of the cornerstones of AH management. AH patients are at high risk of infections (Fig. 3). First, because with fibrosis progression liver architecture is distorted and immune liver surveillance is impaired [75], which provokes a pro-inflammatory state that eventually leads to immune exhaustion [76,77]. Second, alcohol leads to bacterial overgrowth and gut dysbiosis and disrupts gut tight junctions causing a leaky bowel, which stimulates enteric bacteria, pathogen-associated molecular patterns (PAMP) and lipopolysaccharide (LPS) translocation [78,75]. Third, but not last, glucocorticoids, the main treatment for AH, diminish cellular response to pathogens.
For infection screening, a chest x-ray and blood, urine, and ascites cultures should be obtained in the initial work-up. It has been consistently reported in the literature that respiratory and urinary account for most of the infections [75,79]. Respiratory infections accounted for 50 % of the reported in the STOPAH trial (Steroids or Pentoxifylline for Alcoholic Hepatitis) [80]. Remarkably, differences of infection sites before and after glucocorticoids initiation have been described [81]. Before steroids, spontaneous bacterial peritonitis (SBP) and bacteriemia were more frequent (44 %) followed by urinary tract infections (UTI) (32 %). During or after steroids respiratory tract infections (40 %), bacteremia, SBP (28 %) and UTI (18 %) predominated.
Given the fact that 50 % of AH is complicated by infection in the short-term and 25 % present symptoms or signs of infection at admission [81] it has been questioned whether the use of prophylactic antimicrobial therapy may be of benefit. A recent randomized clinical trial conducted in 25 centers in France and Belgium evaluated the prophylactic use of amoxicillin-clavulanate in patients with biopsy-proven severe AH, combined with prednisolone as the mainstay therapy. The group treated with amoxicillin-clavulanate three times daily for 30 days showed a decreased rate of all types of infections. However, the addition of antibiotic therapy did not show a reduction in 2-month mortality, and it had no effect over the therapeutic response to prednisolone, liver function or incidence of hepatorenal syndrome. Therefore, this study does not support the prophylactic use of antibiotics in severe AH [82]. Also, the high prevalence of multidrug resistance organisms and the impact on mortality of cirrhotic patients [83], as well as the increased risk of fungal infections should be considered when starting antibiotics [84].
Nutritional assessment is the other cornerstone in the management of these patients. Malnutrition and micronutrient deficiencies are common among people with AUD and ALD and are directly associated with liver disease severity [85] and survival impairment [86]. A randomized clinical trial demonstrated that the combination of enteral feeding and glucocorticoids was not superior to oral nutrition [87]. This research also highlighted the importance of balanced nutrition, as a low protein-, lipid- and calorie-intake was associated with an increased mortality. Therefore, dietary supplementation is vital, independently of the feeding route, aiming to achieve 35–40 kcal per kilogram of body weight per day.
Acute kidney injury (AKI) is an early complication, common in patients with cirrhosis [88], and bacterial infections [89,90]. AKI has been associated with a higher short-term mortality rate in AH [91]. It is strongly recommended to pay careful attention to even slight variations in creatinine as renal dysfunction adversely affects prognosis.
AUD should not be forgotten when treating patients with AH, as complete alcohol cessation has a positive impact on long-term survival, and it is the best predictive factor of long-term mortality [92]. There is evidence supporting that interventions on AUD may be more effective in patients with ALD after an episode of hospitalization due to decompensation of liver disease [93]. AUD should be evaluated by alcohol specialists. This is reinforced by the retrospective analysis by Peeraphatdit et al. [94] of AH inpatients from 1999 to 2015 that analyzed which factors were associated with readmission rate, relapse, and death. Patients assessed by addiction specialists during hospitalization attended more alcohol rehabilitation than those whose AUD was not assessed (25.3% vs. 10.4 %). Withdrawal syndrome and Wernicke's encephalopathy are two conditions that should be prevented. Severe withdrawal syndrome (CIWA-R > 21 points) [95] requires from intravenous benzodiazepines administration, that are usually contraindicated in these patients because it may precipitate an episode of encephalopathy.
5.3Specific managementSince 1971, corticosteroids [96] have been the only pharmaceutical treatment available that improves survival. Unfortunately, its effect is modest and limited to the first 28 days of treatment. Several scoring systems have been designed to predict mortality in AH and to select patients who could benefit from glucocorticoids. Traditionally, severe AH had been defined as a Maddrey modified discriminant function (mDF) greater than 32 points [96]. Other designed scoring systems are the ABIC score (albumin, bilirubin, INR and creatinine) [97] and the Glasgow score (age, white blood cell count, bilirubin, BUN, PT and PT control) [98]. The Model for End-Stage Liver Disease (MELD) score has also been validated for AH [99]. In 2021, a global survey evaluated the performance of the available scoring systems for AH [100]. This study reported that MELD was the best predictor for short-term prognosis in AH, whereas mDF had the lowest prediction accuracy in comparison to all previously validated scores.
Two large real-world studies have identified a MELD of 21–39 to be the optima therapeutic window for the use of corticosteroids. Similarly, people with AH and a rapid decline in total bilirubin (rapid fallers) do not benefit from corticosteroids [101,102]. When indicated and in absence of contraindications, a 4-week course of 40 mg of prednisone is initiated. The Lille score [103,104] was designed to identify those patients (approximately 40 %) who do not benefit from steroids and avoid futile exposure [103]. A Lille score of >0.45 at day 7 of treatment identifies the non-responders. It has been demonstrated that Lille score calculation on day 4 [105] can also predict those who benefit from steroids.
Different biological molecules have been tested to find new therapeutic strategies for AH. The STOPAH trial [80] compared the effectiveness of placebo versus prednisolone and pentoxifylline, demonstrating that pentoxifylline, a tumor necrosis factor (TNF) inhibitor, did not improve survival and confirmed the short-term effectiveness of prednisolone. Experimental studies have shown a role for TNF in ALD pathogenesis, so some research has been conducted using infliximab or etanercept in AH, but these trials had to be discontinued prematurely due to an increase in mortality [106,107]. N-Acetylcysteine (NAC) has also been tested as a combined therapy for AH. In 2011 a randomized trial [108] was conducted to determine if dual therapy of NAC plus glucocorticoids improved survival. NAC plus glucocorticoids showed a mortality benefit the first month, although the primary endpoint (improving survival at 6-month) was not achieved. Research has been done for the use of colony-stimulating growth factor (C-SGF), showing promising results specially in severely ill patients (median MELD > 25) in the acute phase of the disease, however, no randomized controlled trials have been done in this setting [109].
5.4Early liver transplantation in AHA period of 6-months of alcohol abstinence has been a formal contraindication to LT in patients with ALD including AH. Given the increased availability of organs due to the successful treatment of HCV, the possibility of offering an early LT for patients with severe AH non-responding to medical therapy has been proposed in the last decade. Early LT refers to the LT that is performed without a 6-month period of abstinence. Since the seminal study by Mathurin et al.[110], an increasing number of centers are considering early LT as a rescue therapy for refractory severe AH. Another retrospective study of 147 early transplant recipients for refractory AH in the United States found that 94 % and 84 % of the patients survive 1 year and 3 years, respectively [111]. This study also showed that continued alcohol misuse was infrequent (11 %) but associated with an increased mortality.
Furthermore, the study by Louvet et al.[112] evaluated prospectively the alcohol relapse 2 years after LT, in patients with severe refractory AH and were eligible for early LT compared to a standard LT group in cirrhotic patients after a 6-month period of abstinence. This study showed a higher risk of relapse (RR 1.45 (95 % CI 0·82–2·60)) and a higher rate of high alcohol intake (RR 4.10 (95 % CI 1·56–10·75) in the early LT group. In terms of mortality, the patients in the early LT group showed a 2-year survival of 70 %, compared to an 18 % survival in the historical control group of non-transplanted AH patients. This data confirms the positive effect in mortality by an early LT in selected patients, with the possibility of relapse. More effort is needed regarding addiction management in early LT to improve outcomes.
The decision to indicate an early LT in the setting of AH should be carefully balanced due to the previously discussed circumstances. Moreover, improvement of liver function and resolution of decompensations has been described in patients with ALD listed for LT and recently in patients with severe AH non-responder to corticosteroid therapy considered non-eligible for LT [113].
6Decompensated cirrhosis and hepatocellular carcinomaPatients with ALD cirrhosis may present with cycles of decompensation and re-compensation that reflects period of heavy alcohol intake and abstinence. The clinical management of alcohol-associated decompensated cirrhosis does not differ from other etiologies: decompensation treatment, primary and secondary prophylaxis establishment when indicated and continuing with the HCC screening with biannual abdominal ultrasounds. HCC has a calculated prevalence of 3–10 % in these patients [114]. Although HCC has a lower incidence in ALD associated cirrhosis rather than in metabolic or viral liver disease; it is usually diagnosed as a more advanced stage and consequently with a lower survival rate [115,116].
Once the onset of decompensation occurs, AUD should be addressed promptly to provide the patient an opportunity to recover and cope with a substance abuse disorder that may jeopardize LT if needed. [117].
7Liver transplantationLT is the treatment of choice for alcohol-associated cirrhosis, being nowadays the leading cause of LT in Europe and North America [48]. When assessing the LT candidacy of a patient with ALD, social support evaluation by a specialized team, and a substance use appraisal by addiction specialists, independently of sobriety duration, are mandatory. Most LT centers still require 6 months of abstinence, demonstrated with biomarkers, prior to admission to a LT waitlist (WL). However, there is a current trend to transplant patients with shorter period of sobriety and other favorable predicting factors.
Although patients’ survival rate after LT is similar for alcohol associated etiology to other etiologies [118], concerns of alcohol relapse exist among healthcare providers, because alcohol relapse is associated with graft loss and allograft fibrosis [119,120]. Despite a careful selection of LT candidates’ appropriateness, alcohol relapse occurs and ranges from 15 % to 40 % [46,111,121]. Alcohol toxicity unawareness [122], poor social support [123], psychiatric comorbidities and shorter abstinence duration before LT [124,125] are predictors of recidivism.
Different scores have been developed to objectively assess the risk of alcohol relapse after LT. The High-risk Alcoholism Relapse (HRAR) scale is now validated in the LT setting [126]. According to Lombardo-Quezada et al.[127] an HRAR > 3 may be a useful predictor for a higher risk of harmful drinking in the post-transplant period.
Although the 6-month sobriety rule is arbitrary, this abstinence period could allow patients to recover and avoid LT because of improvement [128] .In a recent European cohort [113], 8.6 % of the patients with alcohol-associated cirrhosis admitted to the WL improved and were delisted. Factors that predicted delisting were low MELD score, high platelet count at admission and feminine sex.
Attention is paid to the risk of relapse in the post-transplant period, albeit causes of death of ALD transplant recipients differ from other etiologies, consisting of de novo malignancies [129], cardiovascular events [130] and social causes [131]. Aerodigestive tract malignancies represent a high proportion of post-LT neoplasms [132]. Besides the role of alcohol carcinogenic compounds and calcineurin inhibitors-related carcinogenesis, ALD patients have higher rates of cigarette addiction that need to be addressed [133].
8Future directionsAlcohol misuse leading to ALD has a devastating impact on an individual's health, and it profoundly affects society health and socioeconomic burden. Different international organizations have called for action by governments to implement public health policies [134] to mitigate and prevent alcohol-associated detriment, as alcohol consumption is expected to increase in the coming years [135]. Detecting ALD at an early stage is crucial given that presently, abstinence is the only proven option that increases the survival of these patients. A multi-stake holistic societal approach is needed to reduce ALD burden.
Author contributionsHelena Hernández-Évole: Formal analysis, Investigation, Visualization, Writing – original draft. Natalia Jiménez-Esquivel: Formal analysis, Investigation, Writing – original draft. Elisa Pose: Data curation, Methodology, Project administration, Resources, Supervision, Validation. Ramon Bataller: Conceptualization, Data curation, Methodology, Resources, Project administration, Supervision, Validation.
FundingRamón Bataller is a recipient of NIAAA grants U01AA021908 and U01AA020821. Elisa Pose is a recipient of an Instituto de Salud Carlos III grant PI22/00910.
Declaration of interestsNone.





![Worldwide prevalence of death rates related with alcohol-associated cirrhosis (with permission from Huang D, et al. [136]. Worldwide prevalence of death rates related with alcohol-associated cirrhosis (with permission from Huang D, et al. [136].](https://static.elsevier.es/multimedia/16652681/0000002900000001/v1_202401030947/S166526812300265X/v1_202401030947/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)
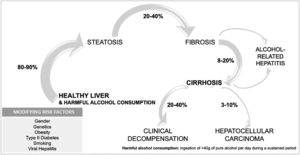 ALD, risk factors, and comorbidity.' title='Spectrum of
ALD, risk factors, and comorbidity.' title='Spectrum of