Sirolimus is an approved anti-rejection agent following liver or kidney transplantation that works through inhibition of the mammalian target of rapamycin (mTOR). As sirolimus functions through a pathway independent of calcineurin inhibition, it may have less potential for nephrotoxicity and carcinogenesis. That being said, there are a myriad of potential adverse effects reported with sirolimus, many of which are severe and unknown or poorly understood. Herein we present a case of sirolimus causing a serious but uncommon adverse event in an adult liver transplant recipient; the adverse event in this instance unfortunately resulted in significant medical testing and morbidity. The adverse event profile of sirolimus is summarized through review of available evidence.
A 65 year-old woman with decompensated cirrhosis secondary to non-alcoholic fatty liver disease (NAFLD) underwent an uneventful liver transplantation (LT). Her medical background was significant for diabetes mellitus controlled with dietary modification, class I obesity, and a remote history of primary bladder cancer (tumor 1, grade 3) treated with intravesical bacillus Calmette-Guerin with no recurrence following serial cystoscopies for 4 years prior to LT. The patient was stable after LT with normal hepatic graft function on a combination of tapering corticosteroids, mycofenolate mofitil (MMF), and tacrolimus. Considering her history of bladder carcinoma, she was switched from tacrolimus to sirolimus (rapamycin) 24 weeks following LT. Four weeks later, the patient developed new-onset ascites, abdominal discomfort, nausea and anorexia. Physical examination revealed stable vital signs, normal jugular venous pulse, tense ascites and moderate bilateral pedal edema. Two sequential paracenteses yielded bloody fluid, and as such the samples were unreliable to assess cell counts and protein, but cytology was negative for malignancy. Over concern of her clinical deterioration and unexplained bloody ascites, a computed tomography scan of the abdomen and pelvis, and cystoscopy, were performed. She was noted to have a lesion suspicious for carcinoma in situ on cystoscopy with biopsy, and cross-sectional imaging showed gross ascites but no obvious masses (or metastases). Due to an absence of evidence for malignancy and no clear cause of the new onset of ascites following a routine work-up, sirolimus was discontinued, and the patient was subsequently converted back to tacrolimus. Six weeks later, the patient’s ascites resolved without intervention. A repeat cystoscopy was normal, with no evidence for any suspicious lesions for recurrent bladder carcinoma. At her last follow-up 1 year upon discontinuation of sirolimus, she was in excellent health.
DiscussionThis case illustrates a severe adverse event secondary to the use of sirolimus in a liver transplant recipient, namely its ability to cause ascites. While sirolimus is not reported to mimic bladder cancer, and the patient’s abnormal biopsy on her initial cystoscopy could well have been a false-positive, we cannot exclude the possibility that sirolimus resulted in irregular bladder wall thickening in this patient, particularly as this finding resolved upon cessation of sirolimus. As a result of this adverse event, our patient underwent unnecessary physical and emotional hardship, as well as a plethora of medical tests, over a legitimate concern of possible recurrence of carcinoma of the bladder. Herein we discuss the use of sirolimus in clinical practice, and review its adverse events, especially as it pertains to liver transplant recipients.
Sirolimus is a macrolide antibiotic originally developed as an antifungal agent, but was later noted to have immunosuppressive and chemotherapeutic properties. Although sirolimus is only approved by the Federal Drug Association as an anti-rejection agent following liver or kidney transplantation, it has a number of off-label uses, including as an anti-rejection agent following heart transplantation, as a prophylactic and/or therapeutic agent for graft-vs.-host disease following allogenic stem cell transplantation, and as a treatment for soft tissue sarcoma.1-3
Sirolimus causes immunosuppression through suppression of T cell activity and antibody production in response to antigenic and cytokine stimulation. These functions are accomplished through the highly specific binding of the drug to the FK506 binding protein 12 (FKBP12) that modulates the activity of the mammalian target of rapamycin (mTOR). mTOR is member of the phosphoinositide 3-kinase related kinase (PIKK) family, and is a principal mediator of cell proliferation. mTOR mediates growth signals through the phosphatidylinositol 3 kinase (Pl3K)/protein kinase B (Akt) signaling pathway, primarily by stimulating downstream protein kinases that are necessary for ribosomal function, and translating essential mRNAs of proteins required for the G(1) to S phase progression in the cell cycle.4 The complex formed between FKBP12 and mTOR prevents cyclin-dependent kinase (cdk) activation, blocks retinoblastoma protein phosphorylation, accelerates the turnover of cyclin D1 that causes a deficiency of active CD-k4/cyclin D1 complexes, and thus inhibits the kinase activity of mTOR, thereby blocking the phosphorylation of key effector molecules, namely p70 S6 kinase (p70S6K) and eukaryotic initiation factor 4E.4,5 The aforementioned mechanisms account for the antineoplastic properties of sirolimus.
As sirolimus works on a pathway independent of calcineurin inhibition, it is believed (by some) to have less potential for nephrotoxicity, and as such, in addition to its theoretical ability to prevent or slow progression of malignancy, many transplantologists prescribe sirolimus to liver and/or kidney graft recipients to avoid nephrotoxicity. Despite published literature and anecdotal experience reporting its renal-sparing properties, a recent systematic review of high-quality observational studies and clinical trials failed to show a statistically significant improvement in renal function in LT recipients switched from a calcineurin inhibitor to sirolimus.6 Furthermore, whilst sirolimus has anti-neoplastic effects in several human cancers and dysfunction of mTOR signaling undoubtedly contributes to cancinogenesis, sirolimus-resistance is frequent in many cancer lines.7-9 Although no mutations in mTOR have been documented in human cancer, alterations of upstream molecules that regulate mTOR and downstream effectors of the mTOR pathway, have been noted.8 Possible mechanisms of resistance to sirolimus include mutations in FKBP12 and other components of the mTOR pathway (e.g. S6K1, 4E-BP1, p27(kip1) and PP2A-related phosphatases.4
Overshadowing the abovementioned important themes of nephrotoxicity and malignancy as it pertains to the use of sirolimus, adverse drug events are frequent and potentially serious. Although calcineurin inhibitors (even at low doses) are also associated with a myriad of potential side effects,10 some of which are severe, the clinician may be appropriately weary of converting a patient to sirolimus if the patient is tolerating calcineurin inhibitors without side effects, unless there is an overwhelming clinical indication to use an mTOR inhibitor in the balance of weighing considerations such as benefit, safety, cost and availability. A systematic review of sirolimus use following LT estimated relative risks of infection at 2.47 (95% confidence interval 1.14-5.36), rash at 7.57 (95% confidence interval 1.75-32.70), ulcers at 7.44 (95% confidence interval of 2.0327.28), and discontinuation of therapy at 3.61 (95% confidence interval of 1.32-9.89).6
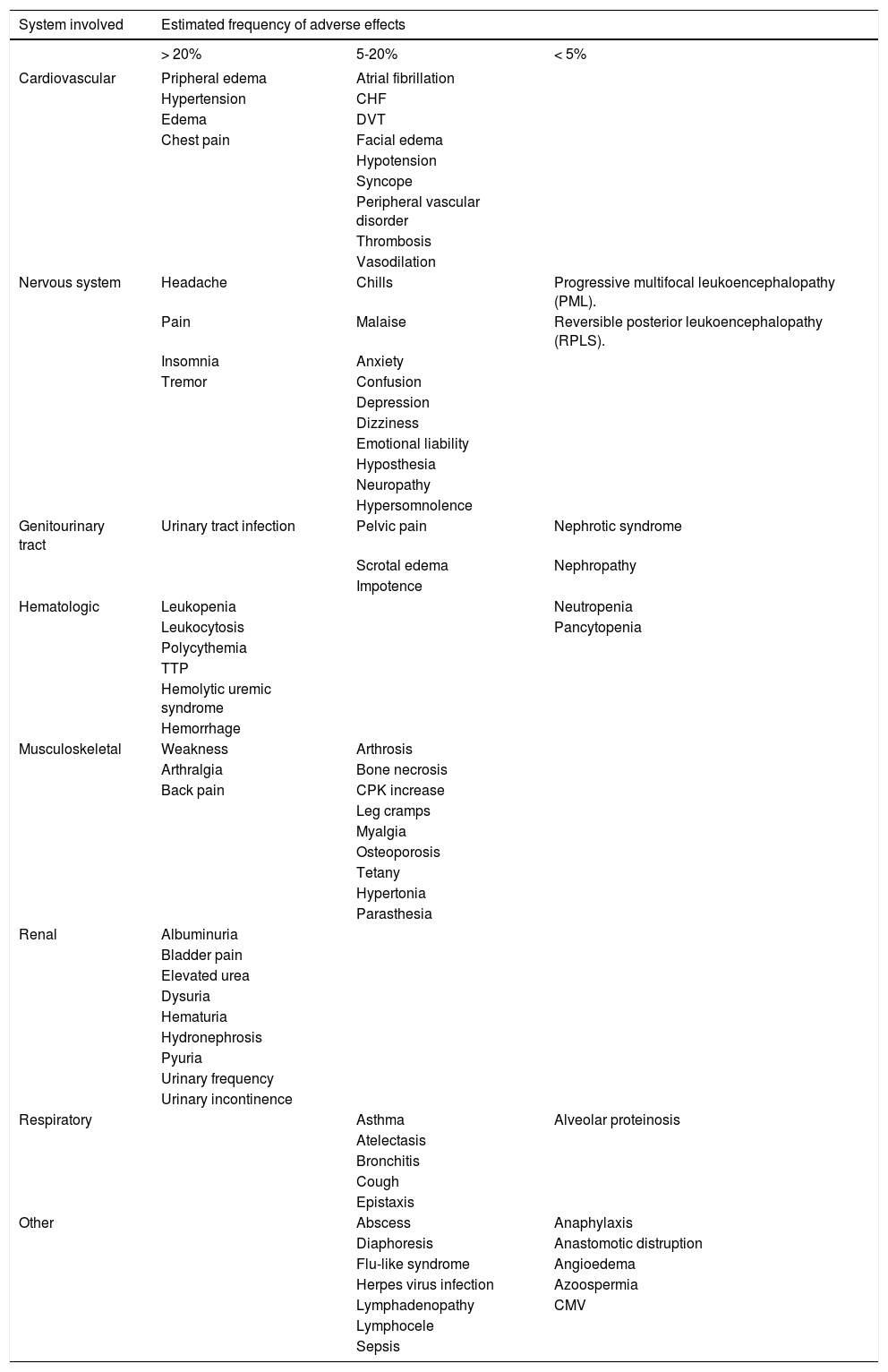
There are a multitude of adverse effects reported with sirolimus, and further data on safety are rapidly emerging given the LT community’s relative inexperience of this agent as compared with other classes of immunosuppressants such as corticosteroids, purine antagonists and calcinurein inhibitors. Examples of frequent adverse events associated with sirolimus include leukopenia, thrombocytopenia, and hyperlipidemia, and less common side effects includes anemia, diarrhea, arthralgias, hypertension and hyperglycemia. Additional adverse events secondary to sirolimus such as hepatic artery thrombosis, lymphocele formation and ascites (both chylous and non-chylous) are reported in the literature, but are considered rare. Unfortunately, the incidence and severity of specific adverse events are difficult to quantitate for clinicians and their patients as the available data relies upon case reports and retrospective experience that are subject to selection and publication biases. A summary of common and uncommon adverse events associated with sirolimus based on the published literature is summarized in table 1.
Common and uncommon adverse events associate with sirolimus.
| System involved | Estimated frequency of adverse effects | ||
|---|---|---|---|
| > 20% | 5-20% | < 5% | |
| Cardiovascular | Pripheral edema | Atrial fibrillation | |
| Hypertension | CHF | ||
| Edema | DVT | ||
| Chest pain | Facial edema | ||
| Hypotension | |||
| Syncope | |||
| Peripheral vascular disorder | |||
| Thrombosis | |||
| Vasodilation | |||
| Nervous system | Headache | Chills | Progressive multifocal leukoencephalopathy (PML). |
| Pain | Malaise | Reversible posterior leukoencephalopathy (RPLS). | |
| Insomnia | Anxiety | ||
| Tremor | Confusion | ||
| Depression | |||
| Dizziness | |||
| Emotional liability | |||
| Hyposthesia | |||
| Neuropathy | |||
| Hypersomnolence | |||
| Genitourinary tract | Urinary tract infection | Pelvic pain | Nephrotic syndrome |
| Scrotal edema | Nephropathy | ||
| Impotence | |||
| Hematologic | Leukopenia | Neutropenia | |
| Leukocytosis | Pancytopenia | ||
| Polycythemia | |||
| TTP | |||
| Hemolytic uremic syndrome | |||
| Hemorrhage | |||
| Musculoskeletal | Weakness | Arthrosis | |
| Arthralgia | Bone necrosis | ||
| Back pain | CPK increase | ||
| Leg cramps | |||
| Myalgia | |||
| Osteoporosis | |||
| Tetany | |||
| Hypertonia | |||
| Parasthesia | |||
| Renal | Albuminuria | ||
| Bladder pain | |||
| Elevated urea | |||
| Dysuria | |||
| Hematuria | |||
| Hydronephrosis | |||
| Pyuria | |||
| Urinary frequency | |||
| Urinary incontinence | |||
| Respiratory | Asthma | Alveolar proteinosis | |
| Atelectasis | |||
| Bronchitis | |||
| Cough | |||
| Epistaxis | |||
| Other | Abscess | Anaphylaxis | |
| Diaphoresis | Anastomotic distruption | ||
| Flu-like syndrome | Angioedema | ||
| Herpes virus infection | Azoospermia | ||
| Lymphadenopathy | CMV | ||
| Lymphocele | |||
| Sepsis | |||
The exact mechanism of ascites formation, as occurred in our patient, is unknown, but proposed theories include capillary leak syndrome (CLS) and prostacycline-induced vasodilatation.6
The use of sirolimus following LT if rife with controversy. Given the importance of the mTOR pathway in carcinogenesis, as well as the high prevalence of calcineurin inhibitor-associated end-stage renal disease after LT,11 it is plausible that sirolimus has a targeted and important role in immunosuppression in liver transplant recipients. That being said, transplantologists and patients alike must be watchful for both common and infrequent adverse events with sirolimus. Our case underscores the need for more prospective safety data on sirolimus to better guide treatment decisions and avoid morbidity after LT.
Conflicts of Interest and DisclosuresNone for all authors.
This submission is original and has not been submitted elsewhere.