Introduction. The inactive hepatitis B surface antigen (HBsAg) carrier state is usually characterized by minimal or absent liver pathology. However, in developing countries, owing to the very early age of infection with hepatitis B virus (HBV), this state is reached after a very prolonged immune tolerant and immune reactive phase, during which considerable liver damage may have occurred. The extent of liver damage in inactive HBsAg carriers has not been thoroughly assessed in developing countries. We thus sought to characterize liver pathology among Egyptian inactive HBsAg carriers.
Material and methods. Liver biopsy was conducted on 30 inactive HBsAg carriers [positive for HBsAg; negative for HBeAg; positive for antibody to HBeAg (anti-HBe); HBV-DNA levels < 2,000 IU/mL; persistently normal serum alanine aminotransferase (ALT)]. Liver histopathology was assessed according to the Ishak scoring system.
Results. Among the studied carriers, 6.7% had no hepatic fibrosis, 73.3% had stage 1 fibrosis, and 20% had stage 2 fibrosis. The majority (80%) of carriers had minimal hepatic necroinflammation (grades 2-4), while 20% had mild hepatic necroinflammation (grade 5). All patients with stage 2 fibrosis were males, while no gender predilection was observed for necroinflammation. Age, ALT and HBV-DNA levels did not differ significantly according to fibrosis or ne-croinflammatory scores. Conclusion. Our study findings do not support the presence of significant hepatic fibrosis or necroinflammation among Egyptian inactive HBsAg carriers. However, follow-up studies on these carriers may be required to monitor any further pathological progress of the disease.
Chronic hepatitis B virus (HBV) infection is an important public health problem, with more than 350 million chronic HBV carriers worldwide.1 Egypt is considered to be a region of intermediate prevalence for HBV infection with a reported figure of 4.5%.2 Chronic HBV infection is a dynamic process, and depends on the interaction between virus replication and host immune response, leading to a replicative or a non-replicative (or low replicative) phase.3 Chronic HBV infection is commonly classified in several phases, which are not necessarily sequential:
- •
Immune tolerant phase characterized by the presence of hepatitis B e antigen (HBeAg), high serum HBV-DNA levels, normal serum amino-transferase levels, and mild or absent hepatic inflammation.
- •
Immune reactive phase which may last from months to years, and where elevation of amino-transferase levels and hepatic inflammation and fibrosis additionally occur. HBeAg seroconver-sion to antibody to HBeAg (anti-HBe) may occur in this phase, and may be associated with biochemical and histological remission of inflammatory activity. The inactive hepatitis B surface antigen (HBsAg) carrier state may follow seroconversion from HBeAg to anti-HBe antibodies and is characterized by very low or undetectable serum HBV-DNA levels and normal serum aminotrans-ferase levels.4 Biopsy findings in this phase can range from mild inflammation and minimal fibro-sis to inactive cirrhosis if the disease was severe during the immune reactive phase.3
Predictors of progression of chronic HBV infection include the duration of the immune reactive phase of the disease that follows the immune tolerant phase.5 Concerning this point, it is well-known that in high endemic areas as Africa and Asia, early perinatal and horizontal infection in childhood are the main routes of HBV transmission, and over 50% of infected cases become chronic HBV carriers.6 Therefore, patients from these areas will have a prolonged immune tolerant phase, followed by an often equally prolonged immune reactive phase, with substantial hepatic inflammation and fibrosis occurring during the latter phase, depending on the degree of host immunity.7 In contrast, HBV infection in low endemic areas as Western countries occurs predominantly in adults, with only 5-10% of infected cases becoming chronic.6 We may therefore hypothesize that a substantially greater degree of liver pathology would be expected in HBV patients from developing countries, as compared to that from Western and developed countries where HBV infection shows much shorter immune tolerant and immune reactive phases. However, to our knowledge, there is very little or no information concerning liver histology among inactive HBsAg carriers in Egypt, where a more considerable degree of hepatic inflammation and fibrosis might be expected, owing to the very early age of onset of infection. Accordingly, we undertook the present study to assess hepatic fibro-sis and necroinflammation among Egyptian inactive HBsAg carriers.
Material and MethodsPatient selectionThe present study was conducted on 30 patients diagnosed as inactive HBsAg carriers, who were recruited from the Hepatology outpatient clinic at Ain Shams University Hospital, Cairo. These patients had been accidentally discovered to be positive for HBsAg for at least one year before recruitment, either after an obligatory health check-up before travelling to a foreign country, during blood donation, during a routine checkup, or during screening of family members of HBsAg positive patients. Patients were asymptomatic, and had no history or clinical evidence of liver disease. Patients were included in the study if they met the following inclusion criteria during enrollment:
- •
Positive for HBsAg at the time of recruitment and for at least the past year.
- •
Negative for HBeAg and positive for anti-HBe.
- •
HBV-DNA levels < 2,000 IU/mL and
- •
Persistently normal serum levels of alanine ami-notransferase (ALT) at the time of recruitment and every 3 months for at least the past year.8
The updated upper limits of normal for ALT levels (30 IU/L for men and 19 IU/L for women) were used.9 Exclusion criteria were:
- •
Co-infection with hepatitis C virus (HCV), human immunodeficiency virus (HIV) or schistoso-miasis.
- •
Alcohol consumption.
- •
Evidence of hepatic decompensation (ascites, jaundice, variceal bleeding, hepatic encephalopa-thy) and
- •
Previous treatment with interferon or antiviral drugs.
In addition, all patients had performed an abdominal ultrasonography at least within the past year, and had no evidence of liver cirrhosis (irregular liver surface, heterogenic texture of liver parenchyma, increased portal vein diameter or splenomegaly). An informed consent was taken from all the study participants after explaining the aim and procedures of the study and ensuring the confidentiality of the data. The study was approved by the Research Ethics Committee and the Review Board of the Gas-troenterology and Hepatology, and Clinical Immunology departments of Ain Shams University Hospital.
Biochemical and serological testsBaseline biochemical tests were performed for all recruited patients, and included serum levels of ALT, aspartate aminotransferase (AST), alkaline phosphatase, bilirubin, total proteins and albumin, which were measured using Beckman-Syn-chron CX5 autoanalyzer (Beckman instruments, Fullerton, CA, USA). Prothrombin time was per-formed using Sysmex CA-1500 analyzer (Dade Behring, Marburg, Germany). A complete blood count was performed using Sysmex KX-21N cell counter (Sysmex Corporation, Mundelein, IL, USA). All patients were tested for schistosomiasis, in addition to HBV serological markers (HBsAg, HBeAg, and anti-HBe), HCV and HIV using commercial kits (DiaSorin S.p.A., Saluggia, Vercelli, Italy). Serum HBV-DNA was determined by poly-merase chain reaction using the QIAamp MinElute Virus Spin Kit (QIAGEN, USA) according to the manufacturer’s instructions.
Liver biopsyA percutaneous liver biopsy was performed for all patients with 16-gauge Tru-Cut needles. The cores were fixed in 10% formaldehyde-saline and processed according to routine histological techniques. Liver histology was graded according to the Ishak, et al. scoring system.10 This system includes a fibrosis score from 0 to 6 and a necroinflammatory score from 0 to 18 [none (0); minimal (1-4); mild (5-8); moderate (9-12); severe (13-18)]. The necroinflam-matory score is the sum of four scores, piecemeal necrosis (0-4); confluent necrosis (0-6); focal lytic necrosis, apoptosis and focal inflammation (0-4); portal inflammation (0-4).
Outcome measuresThe primary outcome measure of the study was assessment of the histological findings of liver biopsy specimens from inactive HBsAg carriers with persistently normal serum ALT levels and low HBV-DNA levels (< 2,000 IU/mL). Secondary outcome measures were possible associations of serum ALT levels and HBV-DNA viral load with the extent of hepatic fibrosis and necroinflammation in these patients.
Statistical analysisAnalysis of data was performed using the SPSS program, version 12. Data were expressed as mean ± standard deviation for parametric data, and as median and interquartile range for non-parametric data, respectively. Parametric data were analyzed using Student’s t-test for the comparison of two groups. Non-parametric data were analyzed using the Mann-Whitney U and Kruskal-Wallis tests to compare two and three groups, respectively. Chi-square test was used to compare categorical data. To assess the correlation between HBV-DNA levels, and the stage of fibrosis and grade of necroinflamma-tion, Spearman’s correlation coefficient was performed. A p-value < 0.05 was considered significant.
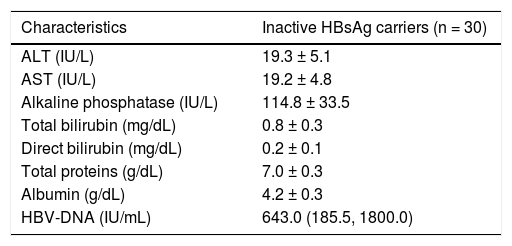
ResultsPatient characteristicsThe present study was conducted on 30 inactive HBsAg carriers (21 males and 9 females) aged 20-44 years (mean = 30.6 ± 6.8 years). All patients were positive for HBsAg for at least 1 year before recruitment, and were asymptomatic with no evidence of liver disease. All patients were negative for HBeAg, positive for anti-HBe, and had HBV-DNA levels < 2,000 IU/mL. None of these patients had shown an increase of serum ALT levels for at least 1 year before recruitment. The biochemical characteristics of these patients during enrollment are presented in table 1.
Biochemical characteristics of the study group.
| Characteristics | Inactive HBsAg carriers (n = 30) |
|---|---|
| ALT (IU/L) | 19.3 ± 5.1 |
| AST (IU/L) | 19.2 ± 4.8 |
| Alkaline phosphatase (IU/L) | 114.8 ± 33.5 |
| Total bilirubin (mg/dL) | 0.8 ± 0.3 |
| Direct bilirubin (mg/dL) | 0.2 ± 0.1 |
| Total proteins (g/dL) | 7.0 ± 0.3 |
| Albumin (g/dL) | 4.2 ± 0.3 |
| HBV-DNA (IU/mL) | 643.0 (185.5, 1800.0) |
All values are presented as mean ± standard deviation except HBV-DNA, as median (interquartile range). ALT: alanine aminotransferase. AST: asparta-te aminotransferase. HBsAg: Hepatitis B surface antigen.
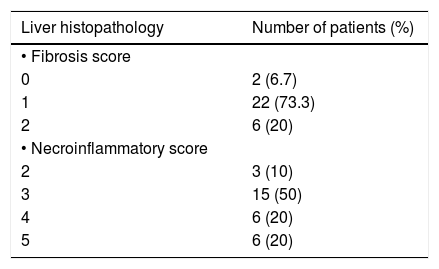
Liver biopsy was performed for all 30 patients after their informed consent, and the Ishak, et al. scoring system10 was used. Two patients (6.7%) had no fibrosis at all, while the majority (73.3%) had a fi-brosis score of 1, and 6 patients (20%) had a fibrosis score of 2. No patient had a fibrosis score of > 2. On the other hand, 3 patients (10%) had a necroin-flammatory score of 2, half the patients (50%) had a score of 3, 6 patients (20%) had a score of 4, and 6 patients (20%) had a score of 5 (Table 2).
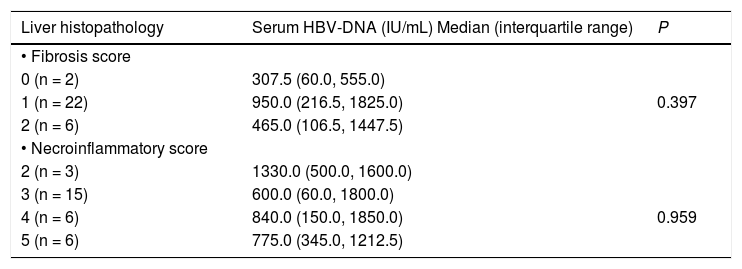
Relation between serum HBV-DNA levels and liver histopathology resultsWe assessed the relation between serum HBV-DNA levels and the various fibrosis and necroinfla-mmatory scores among our patients. As shown in table 3, serum HBV-DNA levels did not differ significantly according to fibrosis or necroinflammatory scores. Furthermore, there was no significant correlation between serum HBV-DNA levels and the stage of fibrosis (r = -0.038, p = 0.842), or between serum HBV-DNA levels and the grade of necroinfla-mmation (r = -0.033, p = 0.863).
Serum HBV-DNA levels according to the liver histopathology results.
| Liver histopathology | Serum HBV-DNA (IU/mL) Median (interquartile range) | P |
|---|---|---|
| • Fibrosis score | ||
| 0 (n = 2) | 307.5 (60.0, 555.0) | |
| 1 (n = 22) | 950.0 (216.5, 1825.0) | 0.397 |
| 2 (n = 6) | 465.0 (106.5, 1447.5) | |
| • Necroinflammatory score | ||
| 2 (n = 3) | 1330.0 (500.0, 1600.0) | |
| 3 (n = 15) | 600.0 (60.0, 1800.0) | |
| 4 (n = 6) | 840.0 (150.0, 1850.0) | 0.959 |
| 5 (n = 6) | 775.0 (345.0, 1212.5) |
According to the Ishak, et al. scoring system.
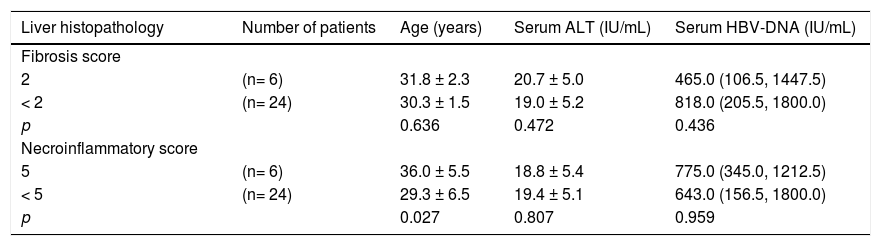
We compared patients who had stage 2 hepatic fibrosis with those who had a fibrosis stage < 2. We also compared patients with mild hepatic necroinfla-mmation (grade 5) with those who had minimal hepatic necroinflammation (grade < 5). Six of the 21 males (28.6%) had a fibrosis score of 2 compared to none of the 9 females (p = 0.073). Three of the 21 males (14.3%) had a necroinflammatory score of 5 compared to 3 of the 9 females (33.3%), p = 0.232. Age was similar between patients with a fibrosis score of 2 and those with a fibrosis score < 2. In contrast, patients with a necroinflammatory score of 5 were significantly older than those with a necroin-flammatory score < 5. Only one patient had a fibro-sis score of 2 as well as a necroinflammatory score of 5. There were no significant differences in serum ALT or HBV-DNA levels between patients with a fi-brosis score of 2 and those with a fibrosis score < 2, or similarly between patients with a necroinflamma-tory score of 5 and those with a necroinflammatory score < 5 (Table 4).
Comparison of age, serum ALT and HBV-DNA levels between patients with different scores of hepatic fibrosis and necro-inflammation.
| Liver histopathology | Number of patients | Age (years) | Serum ALT (IU/mL) | Serum HBV-DNA (IU/mL) |
|---|---|---|---|---|
| Fibrosis score | ||||
| 2 | (n= 6) | 31.8 ± 2.3 | 20.7 ± 5.0 | 465.0 (106.5, 1447.5) |
| < 2 | (n= 24) | 30.3 ± 1.5 | 19.0 ± 5.2 | 818.0 (205.5, 1800.0) |
| p | 0.636 | 0.472 | 0.436 | |
| Necroinflammatory score | ||||
| 5 | (n= 6) | 36.0 ± 5.5 | 18.8 ± 5.4 | 775.0 (345.0, 1212.5) |
| < 5 | (n= 24) | 29.3 ± 6.5 | 19.4 ± 5.1 | 643.0 (156.5, 1800.0) |
| p | 0.027 | 0.807 | 0.959 |
All values are presented as mean ± standard deviation except HBV-DNA, as median (interquartile range). ALT: alanine aminotransferase.
The inactive HBsAg carrier state represents the largest group in chronic HBV infected patients.6 A French study had previously conducted liver biopsy among 58 inactive HBsAg carriers, and concluded that the majority of subjects had either a normal liver histology or a very low histological score, and that only 9% of their studied patients had a fibrosis score of > 2. Furthermore, during a mean follow-up of 3.2 ± 2.6 years in that study, serum HBV-DNA levels remained unchanged and serum ALT levels remained within normal limits in 96% of patients.11 Accordingly, the European Association for the Study of the Liver (EASL) guidelines have concluded that the inactive HBsAg carrier state confers a favorable long-term outcome with a very low risk of cirrhosis or hepatocellular carcinoma (HCC) in the majority of patients.4 However, the appropriateness of these guidelines has not been extensively assessed for developing countries with a higher endemicity of HBV infection.12,13 In these countries, the very early age of onset, and hence the longer duration of HBV infection, may enhance the progression of the disea-se.5,7 In addition, although the prognosis is usually considered to be favorable in the inactive HBsAg carrier state, some patients may still have re-activation of HBV replication, and the occurrence of cirrhosis and HCC has been reported in a prospective Taiwanese study.14 In fact, guidelines have recommended screening for HCC for African HBV carriers older than the age of 20, as a high risk group.15 However, there are few studies to our knowledge which have assessed liver histopathology among inactive HBsAg carriers in developing countries, with almost no studies conducted in Egypt. Our study revealed only mild hepatic fibrosis among the inactive HBsAg carriers; the majority of carriers had stage 1 fibrosis, and 20% had stage 2 fi-brosis. Necroinflammation was mild among 20% of all carriers and minimal among the remainder.
A study in India reported that among inactive HBsAg carriers with persistently normal ALT levels, 21% had histologically active liver disease. In that study, the authors observed that 13.8% of patients with persistently normal ALT levels had significant hepatic fibrosis.12 Another study in Bangladesh reported that 18% of inactive HBsAg carriers had mild degrees of necroinflammation, 26% had moderate degrees of necroinflammation, while 11% were found to have severe hepatic fibrosis.13 Additionally, Papatheodoridis, et al. had studied 35 inactive chronic HBV carriers in Greece. Using the Ishak scoring system, the authors reported that sta-ge 1 hepatic fibrosis was observed in 69%, and stage 2 in 17% of these carriers. Necroinflammatory activity was minimal in 97% of their studied carriers and mild in 3%.16 However, all the inactive chronic HBV carriers in their study had HBV-DNA levels ranging from 2,000 to 20,000 IU/mL, and therefore did not strictly fulfill the American Association for the Study of Liver Diseases (AASLD) criteria for diagnosing inactive HBsAg carriers.8 A study among Egyptian HBV patients with negative HBeAg demonstrated that among patients who had HBV-DNA < 2000 IU/mL and normal ALT levels defined by updated criteria, 16% had significant hepatic fibrosis (Metavir score of ≥ 2).17 However, in contrast to the present study, the authors did not stipulate the presence of persistently normal ALT levels among those patients, and so they could not rigorously assess histopathology results among strict inactive HBsAg carriers.
In the present study, a higher proportion of males had stage 2 hepatic fibrosis compared to none of the females. Although this difference did not reach statistical significance, it demonstrates the male gender predominance usually observed in HBV activity.8,18 The duration of HBV infection is also an important factor which may predict the progression of liver disease, and thus the presence of liver fibrosis. In the present study, we were unable to determine the duration of HBV infection among the inactive HBsAg carriers, as all these carriers had been asymptomatic. However, all our subjects were Egyptians, and HBV infection early in childhood is very likely in Asian and African countries. Therefore, age in the present study may roughly represent the duration of HBV infection. Studies in the literature have shown that older age is an independent predictor of liver fi-brosis.18,19 However, owing to the relatively small number of study participants, we could not observe any significant difference in age between carriers with different stages of fibrosis.
In the present study, we used the updated upper limits of normal for ALT levels (30 IU/L for men and 19 IU/L for women)9 to define carriers with normal ALT levels. ALT levels are commonly used to assess liver disease, and elevated levels have been shown to be associated with active liver disease on histology while normal ALT with inactive histology.7 However, studies have shown that ALT levels may not always show significant correlations with liver fibrosis or necroinflammatory activity.9,20 A recent large study showed that among chronic HBV patients with persistently normal ALT levels, 37% had significant fibrosis or inflammation.19 Within the normal range of ALT levels in our study, no significant associations were observed between serum ALT and either the stage of liver fibrosis or the grade of necroinflammation. This is expected due to the very small range and variation of ALT levels in the study.
Measurement of HBV-DNA is used to evaluate disease activity, assess the efficacy of antiviral therapy, and predict treatment outcomes.7 Several reports have shown a positive relationship between HBV-DNA levels, and hepatic fibrosis and necroin-flammation among HBeAg-negative patients.17,21 Other studies have shown that even among subjects with low HBV-DNA levels, HBV complications and liver damage may arise, independent from other disease factors, especially among populations who acquire the virus early in their life.22 In the present study, no significant correlation was found between serum HBV-DNA levels, and liver fibrosis or necro-inflammation, which has been reported previously among inactive HBsAg carriers.11
In conclusion, our study is one of the very few Egyptian studies to assess liver biopsy findings among inactive HBsAg carriers. The results of the present study demonstrated only mild fibrosis and necroinflammation among Egyptian inactive HBsAg carriers. It is thus possible that only a small degree of liver damage occurs during the relatively prolonged immune reactive phase, confirming the good prognosis of these patients, even in countries with very early onset of HBV infection. According to the findings of various cross-sectional studies conducted in developing countries, several investigators have suggested modifying HBV treatment guidelines for these populations.12,13,17 Al-Mahtab, et al. recommended considering trials on antiviral treatment for asymptomatic HBV carriers.23 However, in light of the present study findings, we believe it is probably more appropriate to conduct a larger scale follow-up study of inactive HBsAg carriers for several years to monitor any pathological progress of the disease. Whether these carriers could benefit from antiviral therapy or not still remains to be elucidated.
Conflict of InterestThe authors declare that no funding or grant was received for the study, and that they have no conflict of interest, financial or personal relationship related to the study.