Background. Treatment of hepatitis C virus (HCV) infection with newer direct-acting antivirals is unrealistic in some countries because of the lack of availability.
Aim. Assess benefits and harms of boceprevir (BOC) and telaprevir (TLV) in treatment of genotype 1 HCV infection, and identifying subgroups with most benefit.
Material and methods. Search from 2009-2013 in PubMed, EMBASE, and “gray literature” of published and unpublished randomized trials reporting sustained viral response (SVR) or adverse events (AE) with BOC or TLV + pegylated interferon and ribavirin (PR) in HCV-infected patients; cohorts or case reports for comparison protease inhibitors (PI), evaluation of predictors of SVR, and resistant variants. Cochrane guidelines were applied. Comparisons between PI + PR vs. PR were performed. Main outcomes were expressed as risk-ratios with 95% CIs. Meta-regression and trial sequential analysis were performed.
Results. 33 studies (10,525 patients) were analyzed. SVR was higher for PI + PR (RR, 2.05; 95% CI 1.70-2.48). In meta-regression, previously treated patients exhibited greater benefit from PI + PR (RR, 3.47; 95% CI, 2.78-4.33). AE were higher with PI + PR (RR, 1.01; 95% CI, 1-1.03; NNH 77.59), also the discontinuation rate (RR, 1.69; 95% CI, 1.36-2.10, NNH, 18). Predictors of SVR were IL-28 TT, nonblack race, low viral load, age, no cirrhosis, statin use, undetectable viral load at the first anemia episode and at week 2 of treatment, and low IL-6 levels. In conclusion SVR was higher in patients treated with PIs, patients previously exposed to PR showed superior response rates. Specific predictors will determine the best candidates for treatments that will offer real-life therapeutic alternatives.
The treatment of hepatitis C virus (HCV) infection has been revolutionized by the advent of new drugs such as direct-acting antivirals (DAAs) including NS3/NS4A serine protease inhibitors (PIs), NS5A inhibitors, nucleoside and nonnucleoside NS5B polymerase inhibitors, and cyclophilin inhibitors. Clinical guidelines have tried to keep pace with these developments and have recommended the use of therapeutic options that produce the largest sustained virological response (SVR), with the lower risk of adverse events (AEs), in the shortest time. Such options include sofosbuvir and newer drugs.1
Although this rationale is impeccable from a therapeutic standpoint, its translation to real-life conditions seems problematic at best. The high cost of the latest therapeutic options represents a major barrier for the adoption of these guidelines in low- and middle-income countries, where more than 80% of the HCV-infected patients live.2 Thus, less effective treatments will be part of common clinical practice in most countries, and high-quality data are needed to offer the best realistic therapeutic options.
Although boceprevir (BOC) and telaprevir (TLV) were approved in 2011, the current guidelines for the treatment of chronic genotype 1 HCV infection no longer recommend their use for the initial treatment or in patients who relapse after treatment with pegylated interferon plus ribavirin (PR).3 Yet, PI+PR and PR alone have produced average SVRs of 67 and 34%, respectively, which might be optimized according to the well-defined predictors of a response to tailor the treatment schema to the best candidates.4,5 Systematic reviews and meta-analyses have demonstrated a higher SVR in genotype 1 patients treated with the first approved DAAs, BOC and TLV.6–8 However, previous studies have fallen short in identifying the best candidates for PI + PR treatment, in formally testing the differences in SVRs between different PIs, and in identifying areas in which more research is needed to personalize treatment and optimize more affordable therapeutic options.
The aim of this systematic review and meta-analysis is to assess the benefits and harms of PIs in the treatment of genotype 1 HCV infection. We focused on identifying subgroups of patients who could benefit the most from this therapeutic approach, and we searched for an evidence-based framework for clinical decision-making that could be applied to those health-care systems to which the current clinical guidelines will not apply. The secondary aims were to analyze differences between PIs and to define opportunity areas for future research.
Material and MethodsTwo authors (N.C.T. and M.C.M.R.) independently performed a comprehensive electronic search of papers published from January 2009 to November 2013 using the PubMed and EMBASE databases to identify any study that included the use of BOC or TLV in combination with PR and that reported the SVR, safety, viral response predictors, resistant variants, or a comparison between the new drugs (BOC and TLV) and dual-therapy (PR). Response-guided therapy was not included.9,10
The key words used for the search were hepatitis C virus genotype 1 infection, boceprevir, telaprevir, protease inhibitor, sustained virological response, adverse event, predictor of response, and resistant-associated variants. The search was limited to the English language and human adults. Manual searches of meeting abstracts from January 2011 from November 2013 were also conducted for The Liver Meeting, The International Liver Congress, Digestive Disease Week, and Annual Scientific Meeting of the American College of Gastroenterology. To evaluate the efficacy of BOC or TLV vs. PR only, randomized clinical trials were included. To compare BOC + PR vs. TLV + PR, randomized clinical trials and cohort studies were included. To assess the safety, predictors of response, resistant variants, randomized trials, cohort studies, and case reports were included. The exclusion criteria included use of a PI other than BOC and TLV, incomplete data, nonconcomitant use of PR, and studies in children, animals, or in vitro. In case of disagreement, the full article was retrieved and inspected independently by the two authors; when disagreement persisted, the authors of the report were contacted. Three authors (N.C.T., M.C.M.R., and V.O.A.) extracted the data of the included trials. In case of disagreement, the decision was made by consensus between the three authors or by contacting the authors of the report. Trials were identified by the last name of the first author and year of publication.
The primary outcome was an SVR, which was defined as a negative viral load at week 12 or 24 after the end of treatment. If both time points were reported, the 24-week viral load was preferred. Secondary outcomes were the frequency and type of AEs, determination of the predictors of SVR, and resistant variants to BOC or TLV.
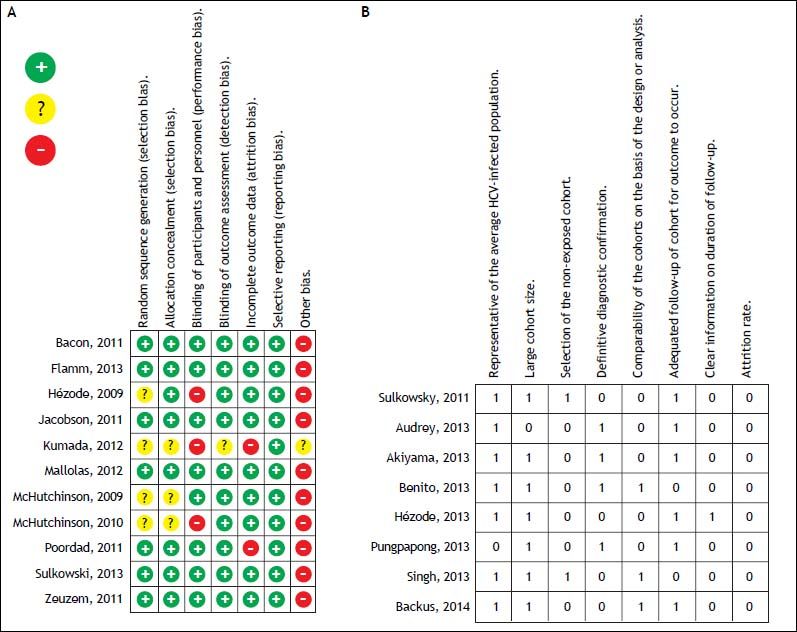
For the quality assessment of the randomized controlled trials (RCT), two authors (N.C.T. and V.O.A.) evaluated the risk of bias of each trial using the Cochrane Collaboration’s risk of bias tool and a predesigned assessment form.11 Seven domains were considered for bias evaluation: sequence generation, allocation concealment, blinding of participants and personnel, outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. The evaluation considered three possibilities: low risk of bias, high risk of bias, or unclear risk of bias. The quality of cohort studies was evaluated by two authors (N.C.T and V.O.A.) by applying the modified Newcastle-Ottawa scale.12 This scale comprises eight items: representativeness of the average HCV-infected population, large sample size, selection of the unexposed cohort, definitive diagnostic confirmation, comparability of the cohorts on the basis of the design or analysis, adequate length of follow-up for outcome to occur, clear information on the length of follow-up, and attrition rate. A score of ≥ 6 points was considered high quality, 4-5 medium quality, and ≤ 3 low quality.
Statistical analysisDifferences in SVR were calculated using intention-to-treat analysis by calculating the risk ratios (RRs) and 95% confidence intervals (CIs). Heterogeneity assessment was conducted using a standard Q test (P < 0.1) and I2 test (>25%). Subgroup analyses were conducted to explore for potential sources of heterogeneity by comparing the use of BOC + PR vs. TLV + PR, naïve vs. previously treated patients, and patients coinfected with human immunodeficiency virus (HIV) vs. non-HIV infected. These factors were then introduced into a meta-regression, with SVR as the outcome, to identify the main sources of heterogeneity while adjusting for covariates (Stata 13.0; StataCorp, College Station, TX). After identification of significant sources of heterogeneity, fixed-effect estimates were calculated in homogeneous subgroups, and random-effects estimates were used to estimate global measures of effect. Data management and analysis were conducted in RevMan 5.2 (The Nordic Cochrane Centre, Copenhagen, Denmark), and the meta-regression was conducted in Stata 13.0.
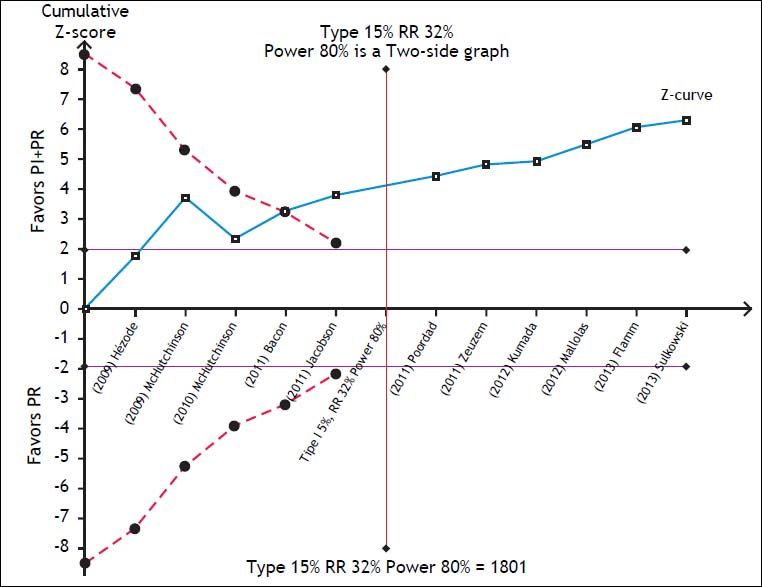
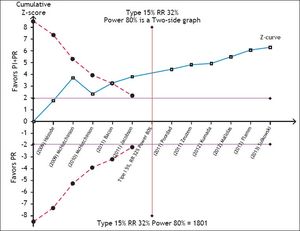
To assess the reliability of the meta-analysis of SVR with PI + PR, the required information size was calculated by trial sequential analysis (TSA, RM5 Converter, the Copenhagen Trial Unit, Center for Clinical Intervention Research, Rigshospitalet, Copenhagen, Denmark).13 We assumed a 32% RR reduction of the experimental intervention and statistical error levels of 5% for alpha and 20% for beta (80% power), respectively. Whenever the cumulative information size in the meta-analysis was smaller than the required information size, the threshold to maintain statistical significance was calculated with the O’Brien-Fleming boundaries.
To identify the most common AEs, the RR, 95% CI, and number needed to harm (NNH) were calculated.
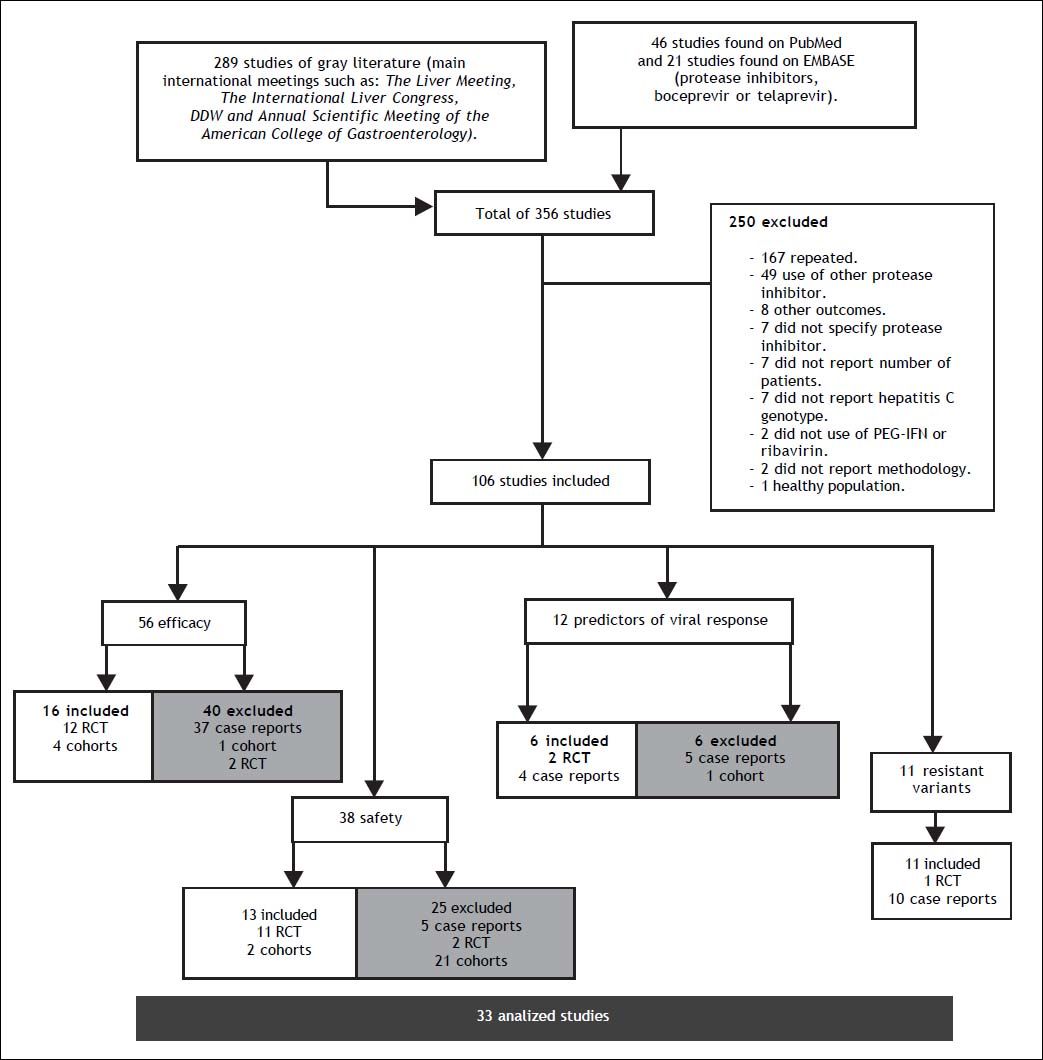
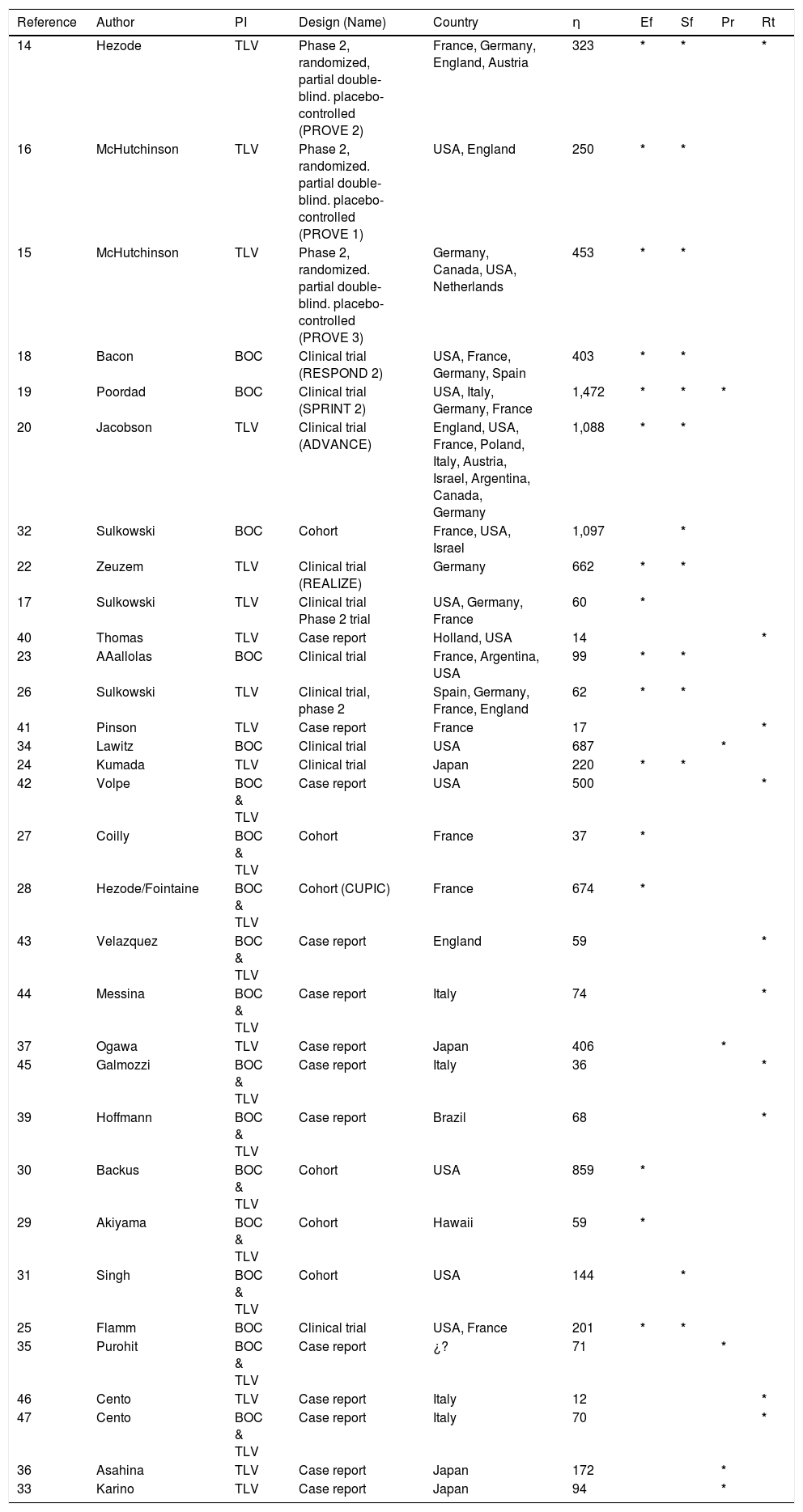
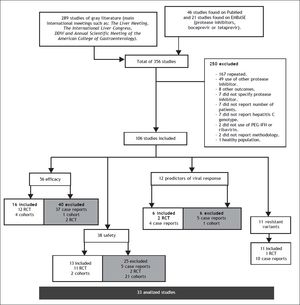
ResultsA total of 356 studies were identified by the initial search strategy, 289 in the supplements of international meetings, 46 studies in PubMed, and 21 on EMBASE. A total of 250 were excluded: 167 were repeated, 49 used a different PI, 8 reported other outcomes, 7 did not report which PI was used, 7 did not report the number of patients, 7 did not report the HCV genotype, 2 did not use concomitant treatment with PR, 2 did not report the methodology, and 1 was conducted on healthy subjects. After a full review of 106 potentially eligible articles, 33 met the inclusion criteria and included a total of 10,525 patients; some of the 33 studies were used for the evaluation of more than one outcome (Figure 1). The characteristics of the included studies are summarized in table 1.
Characteristics of the 33 analyzed studies.
| Reference | Author | PI | Design (Name) | Country | η | Ef | Sf | Pr | Rt |
|---|---|---|---|---|---|---|---|---|---|
| 14 | Hezode | TLV | Phase 2, randomized, partial double-blind. placebo-controlled (PROVE 2) | France, Germany, England, Austria | 323 | * | * | * | |
| 16 | McHutchinson | TLV | Phase 2, randomized. partial double-blind. placebo-controlled (PROVE 1) | USA, England | 250 | * | * | ||
| 15 | McHutchinson | TLV | Phase 2, randomized. partial double-blind. placebo-controlled (PROVE 3) | Germany, Canada, USA, Netherlands | 453 | * | * | ||
| 18 | Bacon | BOC | Clinical trial (RESPOND 2) | USA, France, Germany, Spain | 403 | * | * | ||
| 19 | Poordad | BOC | Clinical trial (SPRINT 2) | USA, Italy, Germany, France | 1,472 | * | * | * | |
| 20 | Jacobson | TLV | Clinical trial (ADVANCE) | England, USA, France, Poland, Italy, Austria, Israel, Argentina, Canada, Germany | 1,088 | * | * | ||
| 32 | Sulkowski | BOC | Cohort | France, USA, Israel | 1,097 | * | |||
| 22 | Zeuzem | TLV | Clinical trial (REALIZE) | Germany | 662 | * | * | ||
| 17 | Sulkowski | TLV | Clinical trial Phase 2 trial | USA, Germany, France | 60 | * | |||
| 40 | Thomas | TLV | Case report | Holland, USA | 14 | * | |||
| 23 | AAallolas | BOC | Clinical trial | France, Argentina, USA | 99 | * | * | ||
| 26 | Sulkowski | TLV | Clinical trial, phase 2 | Spain, Germany, France, England | 62 | * | * | ||
| 41 | Pinson | TLV | Case report | France | 17 | * | |||
| 34 | Lawitz | BOC | Clinical trial | USA | 687 | * | |||
| 24 | Kumada | TLV | Clinical trial | Japan | 220 | * | * | ||
| 42 | Volpe | BOC & TLV | Case report | USA | 500 | * | |||
| 27 | Coilly | BOC & TLV | Cohort | France | 37 | * | |||
| 28 | Hezode/Fointaine | BOC & TLV | Cohort (CUPIC) | France | 674 | * | |||
| 43 | Velazquez | BOC & TLV | Case report | England | 59 | * | |||
| 44 | Messina | BOC & TLV | Case report | Italy | 74 | * | |||
| 37 | Ogawa | TLV | Case report | Japan | 406 | * | |||
| 45 | Galmozzi | BOC & TLV | Case report | Italy | 36 | * | |||
| 39 | Hoffmann | BOC & TLV | Case report | Brazil | 68 | * | |||
| 30 | Backus | BOC & TLV | Cohort | USA | 859 | * | |||
| 29 | Akiyama | BOC & TLV | Cohort | Hawaii | 59 | * | |||
| 31 | Singh | BOC & TLV | Cohort | USA | 144 | * | |||
| 25 | Flamm | BOC | Clinical trial | USA, France | 201 | * | * | ||
| 35 | Purohit | BOC & TLV | Case report | ¿? | 71 | * | |||
| 46 | Cento | TLV | Case report | Italy | 12 | * | |||
| 47 | Cento | BOC & TLV | Case report | Italy | 70 | * | |||
| 36 | Asahina | TLV | Case report | Japan | 172 | * | |||
| 33 | Karino | TLV | Case report | Japan | 94 | * |
TLV: telaprevir. BOC: boceprevir. PI: protease inhibitor, n: number. Ef: efficacy. Sf: safety. Pr: predictors of response. Rt: resistant variant mutations.
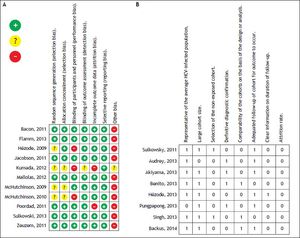
The risk of bias and the quality of randomized trials and cohort studies were classified as a low risk of bias with an unclear selection bias in only 4 studies, and medium quality in most of the cohort studies (Figure 2).
Efficacy studiesA total of 16 studies were included for this outcome; 11 were RCTs14–26 that compared PI + PR with PR, and 4 cohort studies27–30 compared BOC + PR with TLV + PR. A total of 6,922 patients were included in the meta-analysis: 5,293 in RCTs and 1,629 in cohorts.
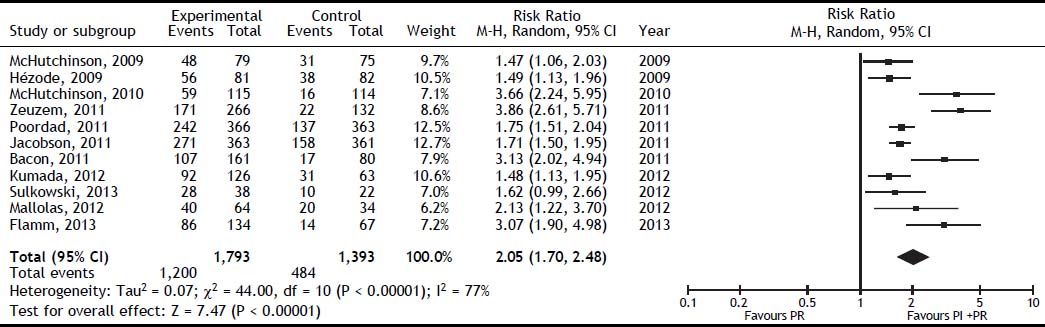
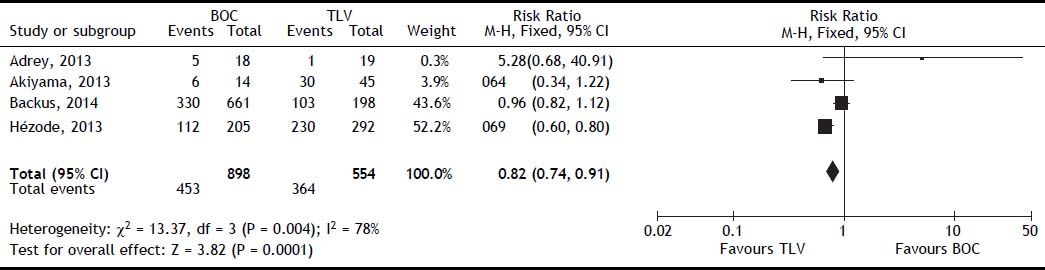
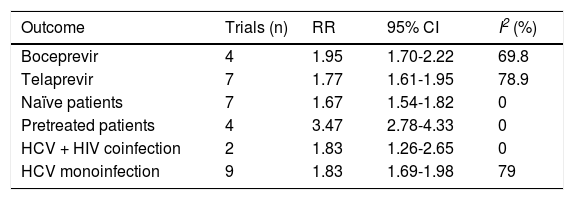
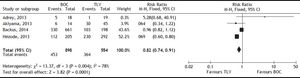
The SVR was higher for PI + PR than for PR (RR, 2.05; 95% CI, 1.70-2.48; I2, 77%) (Figure 3). When analyzed separately, the SVR was higher for BOC + PR (RR, 1.95; 95% CI, 1.70-2.22; I2, 69.8%) and TLV + PR (RR, 1.77; 95% CI, 1.61-1.95; I2, 78.9%) than for PR (Table 2). For the comparison of SVR between BOC + PR vs. TLV + PR, 4 cohorts were analyzed, and a difference on favor to TLV was found (RR 0.82; CI, 0.74-0.91; I2 78%) (Figure 4).
Subgroup analysis of SVR with protease inhibitor plus pegylated interferon plus ribavirin vs. pegylated interferon plus ribavirin.
| Outcome | Trials (n) | RR | 95% CI | I2 (%) |
|---|---|---|---|---|
| Boceprevir | 4 | 1.95 | 1.70-2.22 | 69.8 |
| Telaprevir | 7 | 1.77 | 1.61-1.95 | 78.9 |
| Naïve patients | 7 | 1.67 | 1.54-1.82 | 0 |
| Pretreated patients | 4 | 3.47 | 2.78-4.33 | 0 |
| HCV + HIV coinfection | 2 | 1.83 | 1.26-2.65 | 0 |
| HCV monoinfection | 9 | 1.83 | 1.69-1.98 | 79 |
RR: risk ratio. CI: confidence interval.
In the subgroup analysis, treatment-naïve patients had a lower probability of achieving an SVR in the PI + PR vs. PR comparison (RR, 1.67; 95 CI% 1.54-1.82, I2, 0%) compared with previously treated patients (RR, 3.47; 95% CI, 2.78-4.33; I2, 0%). This result was confirmed in the meta-regression were naïve patients had lower probability of achieving an SVR than did previously PR-treated patients. This is because the SVR rates in PR-treated patients which receive again PR is very low. This difference explains why the RR for PI + PR was larger in previously PR-treated patients than in naïve patients, even while the SVR for PI + PR in previously treated patients (SVR rate, 62%) was similar to that achieved in naïve patients (SVR rate, 70%).
The SVR for the PI + PR vs. PR comparison in HIV-positive and -negative patients did not differ significantly (RR 1.83; 95% CI, 1.26-2.65; I2, 0% and RR, 1.83; 95% CI, 1.69-1.98; I2, 79%, respectively). In the meta-regression, the only significant factor was the previous use of PR; i.e., naïve patients had a 52% lower probability of achieving an SVR than did previously treated patients (95% CI, 0.38-0.61) (Table 2). The TSA showed beneficial effects of the intervention in achieving an SVR, and the cumulative Z-score crossed the O’Brien-Fleming boundaries (Figure 5).
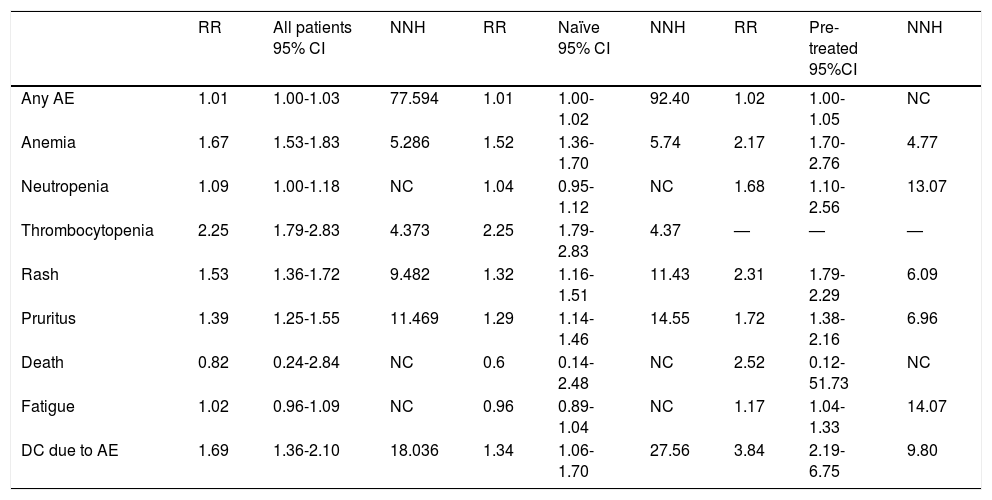
Safety studiesA total of 11 clinical trials14–16,18–20,22–26 and 2 cohort studies31,32 were included in this analysis (6,474 patients). The increased risk of exhibiting any AE with PI + PR vs. PR was low (RR, 1.01; 95%, CI, 1.00-1.03; NNH, 77). The most frequent AEs with PI + PR vs. PR were thrombocytopenia (RR, 2.25; 95% CI, 1.79-2.83; NNH, 4.3), anemia (RR, 1.67; 95% CI, 1.53-1.83; NNH, 5.2), rash (RR, 1.53; 95% CI, 1.36-1.72; NNH, 9.48), and pruritus (RR, 1.39; 95% CI, 1.25-1.55; NNH, 11.46). Less frequent AEs were neutropenia, fatigue, and death. The discontinuation rate because of an AE was higher for PI + PR than for PR alone (RR, 1.69; 95% CI, 1.36-2.10; NNH, 18). When compared the AE between pre-treated patients with PR and naïve patients, the NNH is lower for patients previously treated with PR (Table 3).
Safety analysis of protease inhibitor plus pegylated interferon plus ribavirin vs. pegylated interferon plus ribavirin in all patients, naïve patients, and pre-treated patients.
| RR | All patients 95% CI | NNH | RR | Naïve 95% CI | NNH | RR | Pre-treated 95%CI | NNH | |
|---|---|---|---|---|---|---|---|---|---|
| Any AE | 1.01 | 1.00-1.03 | 77.594 | 1.01 | 1.00-1.02 | 92.40 | 1.02 | 1.00-1.05 | NC |
| Anemia | 1.67 | 1.53-1.83 | 5.286 | 1.52 | 1.36-1.70 | 5.74 | 2.17 | 1.70-2.76 | 4.77 |
| Neutropenia | 1.09 | 1.00-1.18 | NC | 1.04 | 0.95-1.12 | NC | 1.68 | 1.10-2.56 | 13.07 |
| Thrombocytopenia | 2.25 | 1.79-2.83 | 4.373 | 2.25 | 1.79-2.83 | 4.37 | — | — | — |
| Rash | 1.53 | 1.36-1.72 | 9.482 | 1.32 | 1.16-1.51 | 11.43 | 2.31 | 1.79-2.29 | 6.09 |
| Pruritus | 1.39 | 1.25-1.55 | 11.469 | 1.29 | 1.14-1.46 | 14.55 | 1.72 | 1.38-2.16 | 6.96 |
| Death | 0.82 | 0.24-2.84 | NC | 0.6 | 0.14-2.48 | NC | 2.52 | 0.12-51.73 | NC |
| Fatigue | 1.02 | 0.96-1.09 | NC | 0.96 | 0.89-1.04 | NC | 1.17 | 1.04-1.33 | 14.07 |
| DC due to AE | 1.69 | 1.36-2.10 | 18.036 | 1.34 | 1.06-1.70 | 27.56 | 3.84 | 2.19-6.75 | 9.80 |
AE: adverse event. DC: discontinuation. RR: risk ratio. CI: confidence interval. NNH: number needed to harm.
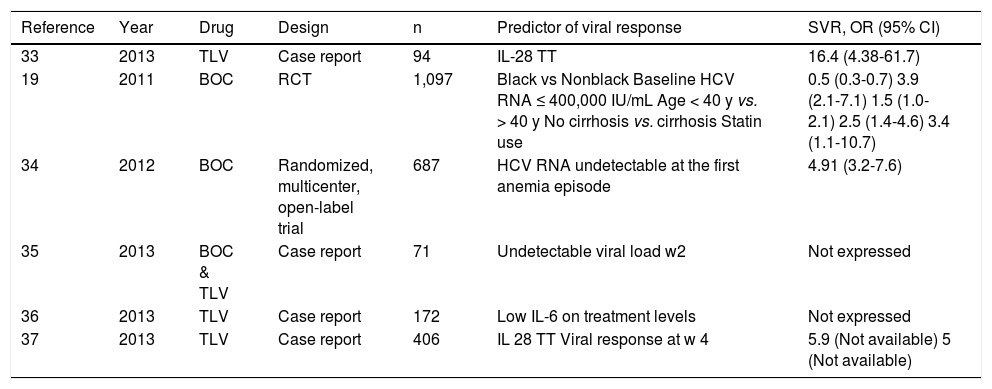
A total of 6 studies and 2,902 patients were included to evaluate the predictors of viral response (4 case reports and 2 randomized multicenter open-label trials).19,33–37 IL-28 genotype TT was reported as a prognostic factor of an SVR in two case report studies by Karino33 and Ogawa37 with an odds ratio (OR) of 16.46 (95% CI, 4.38-61.78; P < 0.001) and 5.93, respectively. In the two randomized multicenter trials, the significant predictors of SVR were: black race (OR, 0.5; 95% CI, 0.3-0.7; P < 0.001), baseline HCV RNA ≤ 400,000 IU/mL (OR, 3.9; 95% CI,% 2.1-7.1; P < 0.001), age < 40 years (OR, 1.5; 95% CI, 1.0-2.1; P = 0.03), no cirrhosis (OR, 2.5; 95% CI, 1.4-4.6; P = 0.003), and statin use (OR, 3.4; 95% CI, 1.1-10.7; P = 0.04). An undetectable RNA viral load at the first anemia episode had a favorable effect on SVR (OR, 4.91; 95% CI, 3.17-7.61; P < 0.0001). Other factors associated with an SVR reported in case reports were an undetectable viral load at week 2 of treatment35 and low levels of IL-6 during treatment36 (Table 4).
Predictors of sustained viral response.
| Reference | Year | Drug | Design | n | Predictor of viral response | SVR, OR (95% CI) |
|---|---|---|---|---|---|---|
| 33 | 2013 | TLV | Case report | 94 | IL-28 TT | 16.4 (4.38-61.7) |
| 19 | 2011 | BOC | RCT | 1,097 | Black vs Nonblack Baseline HCV RNA ≤ 400,000 IU/mL Age < 40 y vs. > 40 y No cirrhosis vs. cirrhosis Statin use | 0.5 (0.3-0.7) 3.9 (2.1-7.1) 1.5 (1.0-2.1) 2.5 (1.4-4.6) 3.4 (1.1-10.7) |
| 34 | 2012 | BOC | Randomized, multicenter, open-label trial | 687 | HCV RNA undetectable at the first anemia episode | 4.91 (3.2-7.6) |
| 35 | 2013 | BOC & TLV | Case report | 71 | Undetectable viral load w2 | Not expressed |
| 36 | 2013 | TLV | Case report | 172 | Low IL-6 on treatment levels | Not expressed |
| 37 | 2013 | TLV | Case report | 406 | IL 28 TT Viral response at w 4 | 5.9 (Not available) 5 (Not available) |
TLV: telaprevir. BOC: boceprevir. IL: interleukin. HCV: hepatitis C virus. y: years. EPO: erythropoietin. RibDR: ribavirin dose reduction. w: week. RCT: randomized control trial.
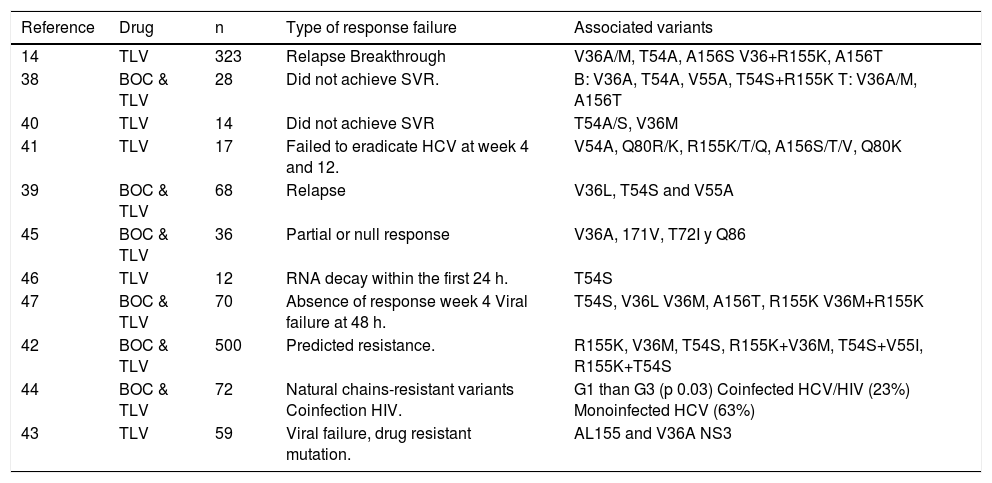
A total of 11 studies reported variants associated with PI resistance: 1 RCT,14 and 10 case reports14,38–47 with a total of 1,255 patients. The virus variants associated with relapse were V36A/M, T54A, and A156S; and for breakthrough V36 + R155K and A156T. Comparative cohort studies showed that the variants associated with BOC resistance were V36A, T54A, V55A, and T54S + R155K and those for TLV were V36M, V36A and A156T. Volpe42 reported resistant mutations for TLV and BOC in 500 clinical samples and found a predetermined resistance in 23% of the patients with viral genotype 1a and 24% with genotype 1b. The most common variants were R155K (12.8%), V36M (11.6%), T54S (4.2%), R155K + V36M (9.8%), T54S + V55I (1.4%), and R155K + T54S (1.6%) (Table 5).
Resistant variant mutations.
| Reference | Drug | n | Type of response failure | Associated variants |
|---|---|---|---|---|
| 14 | TLV | 323 | Relapse Breakthrough | V36A/M, T54A, A156S V36+R155K, A156T |
| 38 | BOC & TLV | 28 | Did not achieve SVR. | B: V36A, T54A, V55A, T54S+R155K T: V36A/M, A156T |
| 40 | TLV | 14 | Did not achieve SVR | T54A/S, V36M |
| 41 | TLV | 17 | Failed to eradicate HCV at week 4 and 12. | V54A, Q80R/K, R155K/T/Q, A156S/T/V, Q80K |
| 39 | BOC & TLV | 68 | Relapse | V36L, T54S and V55A |
| 45 | BOC & TLV | 36 | Partial or null response | V36A, 171V, T72I y Q86 |
| 46 | TLV | 12 | RNA decay within the first 24 h. | T54S |
| 47 | BOC & TLV | 70 | Absence of response week 4 Viral failure at 48 h. | T54S, V36L V36M, A156T, R155K V36M+R155K |
| 42 | BOC & TLV | 500 | Predicted resistance. | R155K, V36M, T54S, R155K+V36M, T54S+V55I, R155K+T54S |
| 44 | BOC & TLV | 72 | Natural chains-resistant variants Coinfection HIV. | G1 than G3 (p 0.03) Coinfected HCV/HIV (23%) Monoinfected HCV (63%) |
| 43 | TLV | 59 | Viral failure, drug resistant mutation. | AL155 and V36A NS3 |
TLV: telaprevir. BOC: boceprevir. NS: not specified. RVR: rapid viral response. G: genotype.
The present systematic review and meta-analysis aimed to assess the benefits and harms of PIs in the treatment of genotype 1 HCV infection. We focused on the identification of subgroups of patients who could benefit the most from this therapeutic approach. The TSA confirmed a higher SVR with the use of PI+PR compared with PR, and meta-regression showed a greater benefit in patients previously treated with PR. The addition of some variables would help in the personalization of PI. This finding should be considered within the context of the problem that newer DAAs are not available or affordable in all countries. Because patients treated previously with PR have a good SVR with BOC or TLV, the initial use of a PR could be feasible, thereby reserving BOC or TLV as an alternative and allowing the newer drugs to be reserved for selected patients. However, the individual patient characteristics should guide the selection of the available therapeutic options.
The TSA on the available data confirmed the certainty about the overall SVR of treatment with PI + PR vs. PR. This may move the analysis to other important outcomes that could help the clinician select the best candidates for this therapeutic approach. In a previous meta-analysis, Park et al suggested that PI + PR therapy may be more effective in patients treated previously with PR than in naïve patients.6 This is surprising considering that the combination of PI + PR should be at least as effective in naïve patients because the effect of a PI should add to the effect of PR. This counterintuitive finding could be attributable to confounding because Park, et al. stratified according to the previous treatment, but did not control for other important factors, such as the presence of HIV infection, kind of PI (BOC or TLV), kind of study (first study or confirmatory study), and whether patients were naïve to PR treatment. For that reason, we conducted a meta-regression to control for these potential sources of heterogeneity. The meta-regression confirmed the results of Park et al because naïve patients had a 52% lower probability of achieving an SVR than did previously PR-treated patients. The explanation for this finding resides in the SVR rates of the control groups, which were treated with PR and not with placebo. PR-treated patients have a very low probability of achieving an SVR with a new round of PR treatment (SVR rate, 17%), whereas naïve patients treated with PR have a much higher probability (SVR rate, 41%). This difference explains why the RR for PI + PR was larger in previously PR-treated patients than in naïve patients, even while the SVR for PI + PR in previously treated patients (SVR rate, 62%) was similar to that achieved in naïve patients (SVR rate, 70%). Therefore, the apparent difference between naïve and previously PR-treated patients was only observable in relative but not in absolute terms.
There was sufficient information to identify differences between patients treated with BOC and TLV, being the patients treated with TLV the most beneficiated when SVR was evaluated, even including the study from Backus30 were an unbalanced in baseline characteristics, particularly for cirrhosis prevalence.
Some predictors of an SVR in patients treated with PI + PR, such as the IL-28b genotype, level of fibrosis, rapid viral response, and viral load, have been reported.7 We also found undetectable HCV RNA at the first anemia episode, undetectable HCV RNA at 2 weeks of treatment, IL-6 levels, and statin use to be important predictors of an SVR. Most of these variables are easily assessed in common clinical practice and could help clinicians individualize treatment.48 However, the quality of the evidence regarding these predictors is limited, and more robust studies may be needed to confirm them.
Finally, we identified important areas for future research. We found 11 studies with the same associated resistance variants as those reported by previous reviews, such as V36A/M, T54A, V55A, R155K/ T/Q, A156S, A156V/T, and V170A for BOC, and V36A/M, T54A, R155K/T/Q, A156S, A156V/T, and V170A for TLV.49 The study of these variants is important, particularly for naïve patients, for whom the presence of resistance variants at baseline can reach 18%, and for HIV coinfected patients who are at increased risk of having one of these variants.50
The overall scenario does not seem as good as that offered with the newer nucleotide analogue NS5B polymerase inhibitors. However, until industry, government, and medical scientific societies provide certainty about the worldwide availability and the prices are affordable for most of the infected populations, most patients will be treated with the currently available options.
Future research is needed to optimize the economic resources. The current recommendations are based on lowering the risk of AEs and providing a better SVR, but there is not enough information about the strategies needed to identify the best candidates to receive PR. Those who have experience treatment failure can be offered PI+PR, and the newer and more expensive drugs could be an alternative for other patients. We consider that 3 years of using the PIs BOC and TLV are of limited experience, and a very difficult scenario to be categorical about the use (or not) of new drugs for the treatment of HCV infection.
This meta-analysis has some limitations. Some of the trials involved selected populations, observational studies were included in the secondary outcomes, heterogeneity could be an issue in some outcomes, and only studies written in English were included.
ConclusionThis systematic review and meta-analysis supports the superiority of any of the first-generation PIs for achieving an SVR in patients with genotype 1 HCV infection. We propose that patients previously exposed to PR are good candidates to receive these drugs. We have identified variables that should help the clinician select the best candidates for newer treatments, which will offer real-life alternatives in scenarios not yet covered by clinical guidelines.
Abbreviations- •
AE: adverse event.
- •
BOC: boceprevir.
- •
CI: confidence interval.
- •
DAA: direct-acting antiviral.
- •
HCV: hepatitis C virus.
- •
HIV: human immunodeficiency virus.
- •
NNH: number needed to harm.
- •
PI: protease inhibitor.
- •
PR: pegylated interferon plus ribavirin.
- •
RCT: randomized controlled trial.
- •
RR: risk ratio.
- •
SVR: sustained virological response.
- •
TLV: telaprevir.
- •
TSA: trial sequential analysis.
This research received no specific grant from any funding agency in the public, commercial or not-forprofit sectors.
AcknowledgmentsNahum Méndez-Sánchez was part of the National Advisory Board of Merck until 2013. All other authors declare no conflict of interest. This work was supported by Medica Sur Clinic & Foundation.