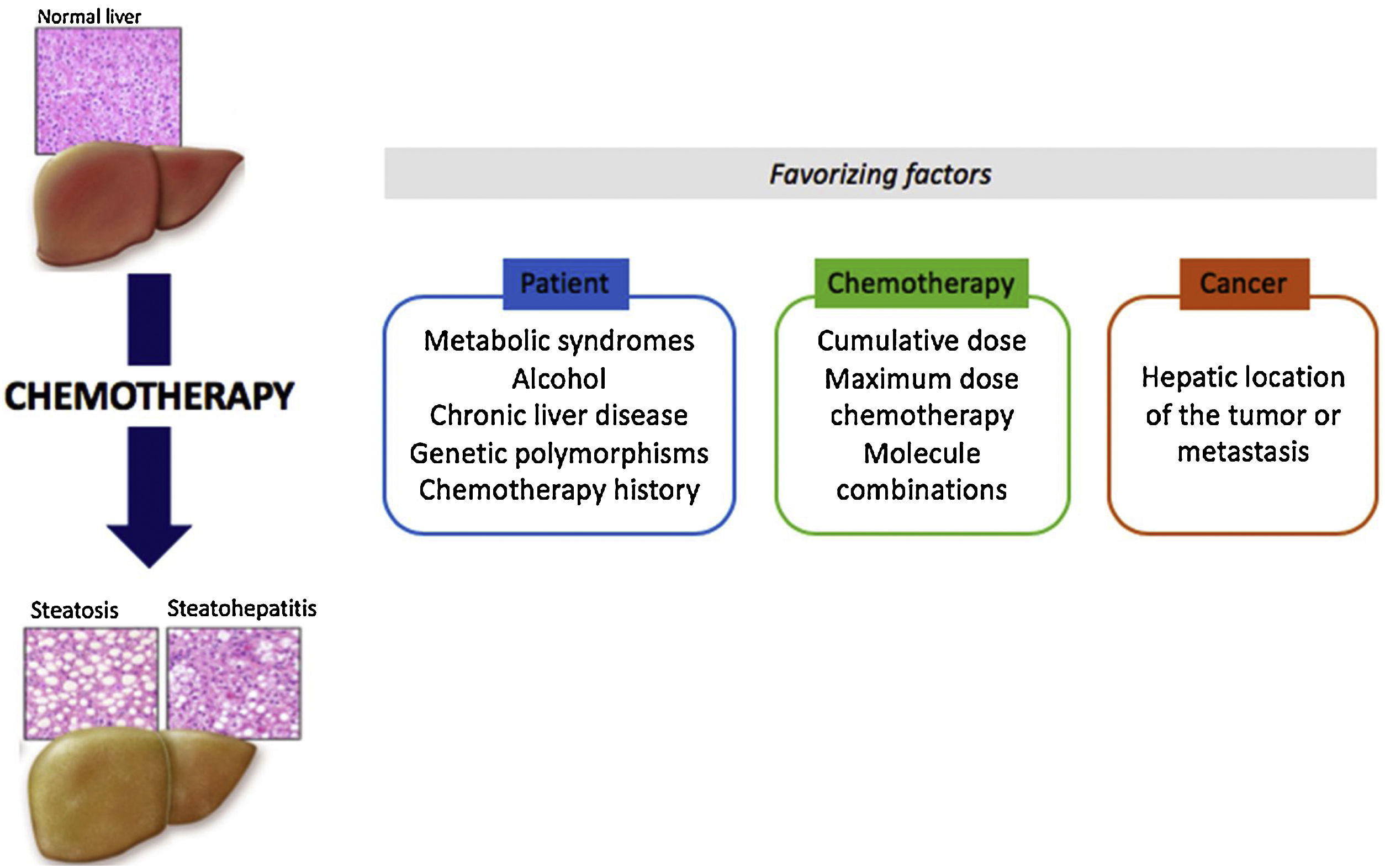
Some drugs may induce hepatotoxic lesions, such as steatosis or steatohepatitis found in Non-Alcoholic Fatty Liver Disease (NAFLD). Among these drugs there are some anti-tumoral molecules, such as methotrexate, 5-fluorouracil, irinotecan, tamoxifen and l-asparaginase. The hepatotoxic phenotype developed from treatment with such drugs is known as “CASH” for “Chemotherapy-induced Acute Steatohepatitis”. The mechanism of toxicity is essentially based on mitochondrial toxicity. These lesions are chronic and often reversible when the treatment is stopped. Contributing factors related to the patient, the disease or the treatment play a major role in the emergence of CASH. It is important to identify chemotherapies with steatosis or steatohepatitis as risk factors in order to improve control of the metabolic risk factors associated with the patient and to reinforce monitoring during treatment. In the particular context of neo-adjuvant chemotherapy for metastatic colorectal cancer, a short duration of chemotherapy and a few-weeks delay between chemotherapy and surgery could reduce postoperative morbidity and mortality.
Drug hepatotoxicity is the main cause of drug withdrawal from the pharmaceutical market and the interruption of new molecule development. Hepatic lesions can vary greatly and reproduce almost all non-iatrogenic liver diseases. Such lesions include steatosis, an accumulation of fat in hepatocytes, and steatohepatitis, steatosis associated with inflammatory foci and enlargement (ballooning) of hepatocytes (Table 1) [1]. Histologically, these drug-related lesions are very similar to the lesions found in metabolic steatohepatitis. These lesions can be caused by various therapeutic agents, including several anti-tumor molecules: methotrexate, 5-fluorouracil, irinotecan, tamoxifen and l-asparaginase (Table 2).
Histological scoring for steatohepatitis diagnosis (NAS score) according to reference [25].
| Steatosis | 0: <5% |
| 1: 5–33% | |
| 2: 33–66% | |
| 3: >66% | |
| Lobular inflammation | 0: none |
| 1: <2 foci per field | |
| 2: 2–4 foci per field | |
| 3: >4 foci per field | |
| Ballooning hepatocytes | 0: None |
| 1: some ballooning hepatocytes | |
| 2: many ballooning hepatocytes |
A score of 0–2 is not in favor of steatohepatitis lesion
A score of 5–8 is compatible with steatohepatitis lesions
Main drugs inducing steatohepatitis lesions according to Ref. [1].
| Chemotherapy | Other drugs |
|---|---|
| Fluorouracil (5-FU) | Amineptin |
| Irinotecan | Amiodarone |
| l-Asparaginase | Aspirin |
| Methotrexate | Didanosine |
| Tamoxifen | Diethylstilbestrol |
| Diethylaminoethoxyhexestrol | |
| Diltiazem | |
| Fialuridine | |
| Glucocorticoids | |
| Hexestrol | |
| Ibuprofen | |
| Nucleoside reverse transcriptase inhibitors | |
| Ketoprofen | |
| Nifedipine | |
| Perhexiline | |
| Pirprofen | |
| Tetracycline | |
| Tianeptine | |
| Valproate |
Chemotherapy-induced steatosis and steatohepatitis are often described by the abbreviation “CASH” for “Chemotherapy-induced Acute Steatohepatitis”. These chronic liver complications may be reversible a few weeks or months after discontinuation of treatment [2]. Conventional imaging methods and liver function tests fail to differentiate between simple steatosis and steatohepatitis. CASH has been particularly observed during chemotherapy for metastatic colorectal cancer [3]. Complicating liver resection outcomes it must be detected before surgery.
2Major drugs involvedTable 3 presents the drugs associated with CASH and the cancers for which they are used.
5-fluorouracil (5-FU) belongs to the chemotherapy class of anti-metabolites (anti-pyrimidines). Uracil, a thymine precursor, is an essential base for DNA synthesis. Thus, 5-FU has several anti-metabolic effects by blocking the synthesis of DNA, proteins and enzymes. 5-FU is one of the most frequently prescribed chemotherapy molecules, particularly for digestive tract head and neck, ovary and breast cancers. It is a rather well-tolerated drug, with severe toxicity rates (neutropenia, mucositis and diarrhea) below 5%. Regarding the liver, 5-FU causes steatosis [4]. This liver side effect is particularly described in metastatic colorectal cancer [5]. Indeed, steatosis incidence varies from 30% to 47% in patients having received 5-FU-containing chemotherapy for liver metastases resulting from colorectal cancer [5]. In a recent published study about gastric cancer, chemotherapy (5 FU) was identifed as an independent risk factor for NAFLD one year after gastrectomy [6].
2.2IrinotecanIrinotecan is a cytotoxic anti-tumor molecule of the DNA topoisomerase inhibitor class. It is metabolized by carboxyl esterase into an active metabolite, SN-38, which has been shown to be more active than irinotecan at inhibiting topoisomerase I. Inhibition of DNA topoisomerase I by irinotecan, or SN-38, induces single-stranded DNA lesions responsible for cytotoxic activity. Irinotecan is mainly used in combination with other molecules (oxaliplatin, 5-FU) in the treatment of digestive cancers (colorectal, pancreatic, etc.). The undesirable toxic effects of irinotecan are mainly diarrhea and agranulocytosis, but it can also cause steatohepatitis lesions [4]. One study has reported that about 20% of patients developed steatohepatitis following neoadjuvant treatment for liver metastases [7,8].
2.3TamoxifenTamoxifen is an anti-estrogen molecule that acts by the competitive inhibition of estradiol binding to its receptors. It is mainly used as an adjuvant treatment for breast cancer. Approximately 40% of patients treated long-term with tamoxifen develop hepatotoxicity in the form of steatohepatitis [9,10]. The median time of tamoxifen-induced steatosis lesion onset is 22 months, but steatosis lesion incidence before 2 years is no different to placebo [10,11]. Several studies have shown that a pre-existing metabolic syndrome and diabetes are predisposing factors for developing steatosis with tamoxifen [10–13]. During tamoxifen therapy, liver enzymes should be monitored regularly. Differential diagnosis between breast cancer liver metastases and pseudo-nodule steatosis lesions can be difficult using imagery. In some cases a liver biopsy is necessary. Letrozole is a treatment of the anti-aromatase class of drugs, also used in breast cancer treatment, and sometimes associated with an increase in transaminases. Unlike tamoxifen, letrozole does not induce CASH [4].
2.4MethotrexateMethotrexate is a folic acid antagonist that inhibits tissue cell proliferation. It is used for its anti-tumor action in head and neck, bladder, gynecologic, pulmonary and hematological cancers (leukemias and lymphomas), but also in many autoimmune diseases, such as psoriatic arthritis, rheumatoid arthritis and Crohn's disease. Methotrexate can cause bland macrovacuolar steatosis, steatohepatitis, fibrosis and cirrhosis. In a large literature review based on treatment of psoriatic arthritis with methotrexate, the presence of fibrosis ranged from 5.7% to 71.8% depending on the study. The risk of hepatotoxicity is correlated with the cumulative dose of methotrexate. Recommendations have been established for liver test monitoring during long-term methotrexate treatment by the medical rheumatological community [14]. The frequency of monitoring varies from 2 to 8 weeks depending on the country, with weekly monitoring in the first few weeks. Liver biopsy has been recommended in many situations before the introduction of methotrexate treatment (chronic alcohol consumption, chronic viral infection or abnormal liver function tests). Biopsies are now increasingly replaced by non-invasive tests such as elastography (FibroScan) [15].
2.5l-Asparginasel-Asparaginase is a protein-based enzyme extracted from Escherichia coli cultures. Its anti-tumor action is achieved through the hydrolysis of and thus depletion of asparagine, an amino acid that cannot be synthesized endogenously by leukemic cells. It is used in the treatment of acute lymphoblastic leukemias. l-Asparaginase is associated with hepatic steatosis in 40–87% of patients up to 9 months after the last injection. The effect of steatosis on morbidity and mortality in patients treated with l-asparaginase for acute lymphoblastic leukemia was found to be non-significant [4,16,17].
2.6GlucocorticoidsGlucocorticoids are not primarily used for anti-tumor purposes, but are sometimes combined with other chemotherapies, such as induction treatments for acute leukemias. High or low doses of glucocorticoids for a prolonged period of time may induce macrovesicular steatosis [4].
2.7Mechanisms of hepatotoxicity (Fig. 1)CASH is based on mitochondrial function alterations. The β-oxidation of fatty acids takes place in the mitochondria and in peroxisomes. Some treatments can induce steatosis by decreasing fatty acid β-oxidation, thus generating oxidative stress via reactive oxygen species (ROS) and their accumulation in hepatocytes [18]. Inhibition of mitochondrial β-oxidation or the carnitine shuttle that delivers medium and long chain fatty acids across the inner membrane into the mitochondria matrix leads to hepatic steatosis and/or hepatotoxicity. For irinotecan and methotrexate, the toxicity mechanism also involves an alteration of lysosomal phospholipid metabolism, which activates the adenosine pathway and increases fatty acid synthesis and coenzyme A sequestration. For tamoxifen, the accumulation into mitochondria inhibit β-oxidation, ATP synthase and respiration. Women with the A2 allele of CYP17A1 have an increased risk of hepatic steatosis after tamoxifen treatment for breast cancer, although how this is related to tamoxifen metabolism is unknow. Finally, all chemotherapy agents can have a direct action on the mitochondria via cytotoxic activity [19].
Mechanism of CASH toxicity [17,18].
Chemotherapy-induced steatosis or steatohepatitis lesions are very similar to metabolic steatohepatitis lesions (NAFLD). Thus, obese and diabetic patients, or patients suffering from other dysmetabolic factors, are all the more at risk of developing NAFLD lesions during chemotherapy with the molecules mentioned above. For example, in the case of 5-FU treatment, obesity and diabetes are independent factors that increase complication risks [5].
Other hepatic comorbidities, such as alcohol or hepatic viruses, may promote chemotherapy-induced liver damage.
For some molecules, genetic polymorphisms are associated with a higher risk of liver toxicity. This has been discussed for tamoxifen and CYP17 genetic polymorphisms [20], as well as for methotrexate and polymorphisms of the methylenetetrahydrofolate reductase (MTHFR) gene. On the other hand, genetic deficiency in LAL (lysosomal acid lipase) does not appear to be involved in methotrexate and tamoxifen hepatotoxicity (personal data based on a small number of patients). Finally, anti-cancer therapeutics are often a combination of several anti-tumor molecules (FOLFIRINOX: 5-FU, irinotecan and oxaliplatin) of which hepatotoxic effects can be combined. Similarly, sometimes in one same patient, several successive chemotherapy lines are proposed, leading to an increase in hepatotoxicity lesions.
Outcome patient when in case of CASH is not known except for tamoxifen and methotrexate. For these drugs, a relationship between prognosis and occurrence of NAFLD has been established [13].
2.9Chemotherapies and colorectal cancer liver metastasesColorectal cancer is the third most common cancer in men and the second most common in women, with 1.4 million new cases worldwide per year. Twenty-five percent of patients have hepatic metastases at diagnosis and 25% develop metachronous metastases. Surgical resection of liver metastases is the standard treatment, but only 20% of patients are immediately eligible for surgery. Liver resection after several potentially hepatotoxic chemotherapy courses may lead to postoperative liver failure [3]. The major challenges of chemotherapy for metastatic colorectal cancer are the prediction and prevention of postoperative morbidity and mortality [3].
3Postoperative complication prevention3.1Duration of chemotherapyPostoperative morbidity is correlated to the duration of preoperative chemotherapy; more than 6 cycles of chemotherapy increases postoperative morbidity (>6 cycles 54% (13/24) vs <6 cycles 19% (4/21), p=0.047) [21]. Similarly, another study found that patients who received more than 12 cycles of 5-FU, with or without oxaliplatin, had an increased risk of requiring a second surgery, and a longer hospital stay, than those who received less than 12 cycles [22]. The most protective duration of chemotherapy to achieve maximum anti-cancer effects with limited hepatotoxicity is a maximum of 4 months [7,23].
3.2Interval between chemotherapy and surgerySeveral studies have shown that a long interval between chemotherapy and hepatic resection reduces hepatotoxicity and the rate of postoperative complications. However, this long interval should be balanced against the risk of tumor progression during the untreated period. Indeed, it has been shown that 4 weeks apart is associated with more complications than 5–8 or 9–12 weeks apart (11% vs 5.5% vs 2.6%, p=0.009) [22].
3.3PharmacogeneticsIrinotecan has a narrow therapeutic index and its toxicity can be prevented by analyzing individual patient pharmacokinetics and pharmacogenetics. An assay based on CYP3A activity reduced inter-individual variations in pharmacokinetics, and thus decreased the formation of one of the main active metabolites, SN-38, and thus the incidence of deep neutropenia. SN-38 is also metabolized by the UGT1A1 enzyme, which allows its inactivation by glucuronidation. Some UGT1A1 polymorphisms are associated with fewer side effects, such as diarrhea and neutropenia [24,25].
4In practice4.1Before treatmentBefore starting CASH-risk chemotherapy, liver function should be assessed, and factors that may induce or accelerate the hepatotoxicity of the chemotherapy should be identified.
The evaluation of liver function before treatment requires at least an analysis of biological liver tests, if possible imaging, especially in patients with metabolic risk factors. In the case of suspected liver disease, the evaluation of fibrosis by non-invasive measures is justified. Liver biopsy may be proposed, particularly in the event of contraindication treatment for hepatic reasons.
All patients should be screened for dysmetabolic risk factors, namely diabetes, high blood pressure, overweight-obesity and dyslipidemia. Other causes of liver disease should also be investigated (alcohol, viral hepatitis, hemochromatosis and other rarer causes).
It is also necessary to look among the patient's usual treatments for those that potentially can induce steatosis or steatohepatitis, and that may constitute a confounding diagnostic factor (Table 2) or other forms of hepatotoxicity. In the event of liver damage, discontinuation of treatment should be discussed.
Finally, in the case of previous chemotherapy, the specific hepatic risk of each treatment and the time to introduce a new therapeutic line should be clearly identified.
4.2During treatmentMonitoring of liver function tests must be carried out especially before each new chemotherapy course. In the case of an increase in transaminases, discontinuation of treatment should be discussed if this increase is due to treatment. In some cases, the risk-benefit balance may encourage further treatment. Herein, the liver evaluation must be done during the treatment, but also at the end of the treatment, especially if liver surgery is planned.
5ConclusionCASH may be induced by certain chemotherapies, or related treatments, such as methotrexate, tamoxifen, 5-FU, irinotecan, l-asparaginase and glucocorticoids. CASH is generally reversible when the causative chemotherapy is stopped. In some cases (breast cancer, metastatic colorectal cancer), the benefit/risk balance is not in favor of stopping treatment. Therefore, it must be possible to anticipate and prevent postoperative complication risks, particularly during liver resection. Many factors must be taken into account to predict the risk of CASH, some related to the patient (metabolic risk factors, pharmacogenetics, chemotherapy history, etc.), and others related to the treatment itself (combination of several hepatotoxic molecules, cumulative dose, etc.).
Significant consequences of CASH on overall patient morbidity and mortality are rare. However, the increased frequency of steatohepatitis risk factors, such as obesity and metabolic syndromes, should lead to a careful risk assessment of the drugs susceptible to cause CASH.AbbreviationsNAFLD
Non-alcoholic fatty liver disease
CASHChemotherapy-induced acute steatohepatitis
5-FU5-fluorouracil
ROSreactive oxygen species
MTHFRmethylenetetrahydrofolate reductase
LALlysosomal acid lipase






![Mechanism of CASH toxicity [17,18]. Mechanism of CASH toxicity [17,18].](https://static.elsevier.es/multimedia/16652681/0000001900000006/v2_202209140825/S1665268120300041/v2_202209140825/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)





