Background and aim. The combination of Sofosbuvir (SOF) and Ledipasvir (LDV) has been lead to considerable enhancement of treatment of hepatitis C virus (HCV) genotype 1 infection. A meta-analysis of the currently available studies was undertaken with the aim to evaluate the antiviral efficacy of SOF/LDV therapy for 12 or 24 weeks with or without Ribavirin (RBV) in patients with HCV genotype 1 infection.
Material and methods. In this meta-analysis, we searched databases including PubMed, Scopus, Science Direct and Web of Science using appropriate keywords. All papers which evaluated the efficacy of combination therapy of SOF/LDV with or without RBV for 12 or 24 weeks among patients with HCV genotype 1 infection were included.
Results. The 20 published articles were assessed for eligibility and finally 10 articles pooling 2248 participants were included in this meta-analysis. Pooled SVR12 for four SOF/LDV regimens were 95% (95%CI = 93%-97%) for 12 weeks of treatment with SOF/LDV, 97% (95%CI = 95%-98%) for 24 weeks of treatment with SOF/LDV, 96% (95%CI = 94%-97%) for 12 weeks of treatment with SOF/ LDV/RBV and 98% (95%CI = 97%-99%) for 24 weeks of treatment with SOF/LDV/RBV. Only in treatment regimen of SOF/LDV for 12 weeks, cirrhosis had a significant effect on the SVR12 (OR = 0.21, 95%CI = 0.07-0.66). Furthermore, NS5A resistance-associated substitutions at baseline were associated with decrease in the rate of SVR (OR = 0.31, 95%CI = 0.2-0.5).
Conclusions. The Interferon-free regimen of SOF/LDV for 12 or 24 weeks with or without RBV is highly effective for treatment of patients with HCV genotype 1 infection.
Among the approximately 80 million individuals in the world with chronic hepatitis C virus (HCV) infection, about 46% are infected with HCV genotype 1, the most difficult-to-treat with interferon-based regimens.1 Because of the low rate of treatment success and expected side-effects of Pegylated-Interferon (PegIFN) and Riba-virin (RBV) for treatment of HCV-infected patients, efforts have been made to develop IFN-free treatment regimens.2,3
Protease inhibitor (PI)-containing regimens for patients with HCV genotype 1 infection was introduced in 2011. However, there have been some drug-drug interactions associated with PI-containing regimens and also they should be used with PegIFN and had many side-effects.4 The treatment of patients with HCV genotype 1 infection has been revolutionized by introduction of recent direct-acting antiviral agents (DAAs) including Sofosbuvir (SOF); a uridine nucleotide analogue inhibitor of the HCV NS5B polymerase and Ledipasvir (LDV); an inhibitor of the HCV-encoded NS5A protein.5,6 The best treatment option is the one which has the highest sustained virologic response (SVR) rate with minimal adverse effects in the shortest treatment duration. The combination of SOF/LDV for 12 or 24 weeks with or without RBV has enhanced the SVR rate up to 95%-100% in treatment-naïve and IFN-experienced patients with HCV genotype 1 in-fection.7 There is a need for determining exact SVR rate for treatment with SOF/LDV in combination with or without RBV in duration of 12- or 24-week. Moreover, effect of some factors such as cirrhosis, previous history of treatment, and NS5A resistance-associated substitutions (RASs) on the SVR rate with the mentioned regimen should be evaluated.
A meta-analysis of the currently available studies was undertaken with the aims of evaluation of the antiviral efficacy of SOF/LDV combination therapy for 12 or 24 weeks with or without RBV in patients with HCV genotype 1 infection.
Material and MethodsData resources and search strategiesIn this meta-analysis, we comprehensively and systematically searched electronic databases including PubMed, Scopus, Science Direct and Web of Science using appropriate search strategies for each database. Focus of the keywords in our search strategies were on the treatment protocols; SOF, LDV and their appropriate alternatives. Furthermore, for finding any possible existing grey literatures we did a search on Google scholar and after finding related titles, we continued our search until we found 200 unrelated serial titles. Moreover, references of the retrieved publications were also searched for identification of any possible missed publications in electronic search. Our last search was performed on September 2, 2015 and no language limitation was considered. An update in our search was performed in March 16, 2016.
Eligibility criteriaAny paper which evaluated the effect of combination therapy with SOF/LDV with or without RBV for 12 or 24 weeks on SVR, 12 weeks after termination of treatment (SVR12) among patients with HCV genotype 1 infection were included in this meta-analysis. The studies with the data for intention-to-treat analysis were included otherwise the paper was excluded. Following items were considered as our exclusion criteria; patients on hemodialysis, patients with previous history of SOF-based treatment, patients with simultaneous infection with human immunodeficiency virus (HIV), patients with decompensated cirrhosis (Child-Pugh B and C8) and history of liver or kidney transplantation.
Study selection, quality assessment and data extractionBased on the PRISMA guideline for reporting of systematic review,9 all papers from search results were independently reviewed by two people (MSR-Z and KH) at each level of screening (title, abstract and full-text). At the end of each level of screening, any disagreements between these two authors were resolved by mutual discussion. However, remained disagreements were resolved by consensus and discussion with other colleagues (BB, SMA and HS).
Cochrane’s risk of bias assessment tool was used for quality assessment of each included paper10 and following bias risks were evaluated; random sequence generation (selection bias), allocation concealment (selection bias), blinding of participant and personnel (performance bias), blinding of outcome assessment (detection bias), co-interventions, incomplete outcome data (attrition), selective reporting (reporting bias), intention-to-treat analysis, group similarity at baseline, compliance, timing of outcome assessments and other biases. Based on this assessment, each bias risk for each paper rated as high, unclear and low. High and unclear risks were scored as zero and low risk as one. Articles with score of more than 6 were categorized as low risk studies. Again any differences or disagreement were resolved by mutual discussion.
Following data for participants of each arm of the included studies were extracted; gender, HCV genotype, cirrhosis, polymorphisms near IFNL3 (rs12979860), history of previous treatment, treatment duration, age, body mass index (BMI) and HCV RNA level before treatment. Furthermore, some data for each study including publication year, sample size and country name were gathered.
Data analysisConfidence interval (CI) for SVR rate in each study was calculated based on the jeffreys method and average amount of upper and lower limits of CI was considered as point estimation for SVR.11 Heterogeneity test was performed using χ2 and I-squared (ranges from 0% to 100%). P value less than 0.1 was considered statistically significant for χ2. According to the result of heterogeneity test, we used fixed- or random-effect models for determining pooled SVR12, 95% confidence interval and P value. Trim and fill method was applied for overcoming possible existing publication bias.12 STATA 10 was used for performing all parts of data analysis.
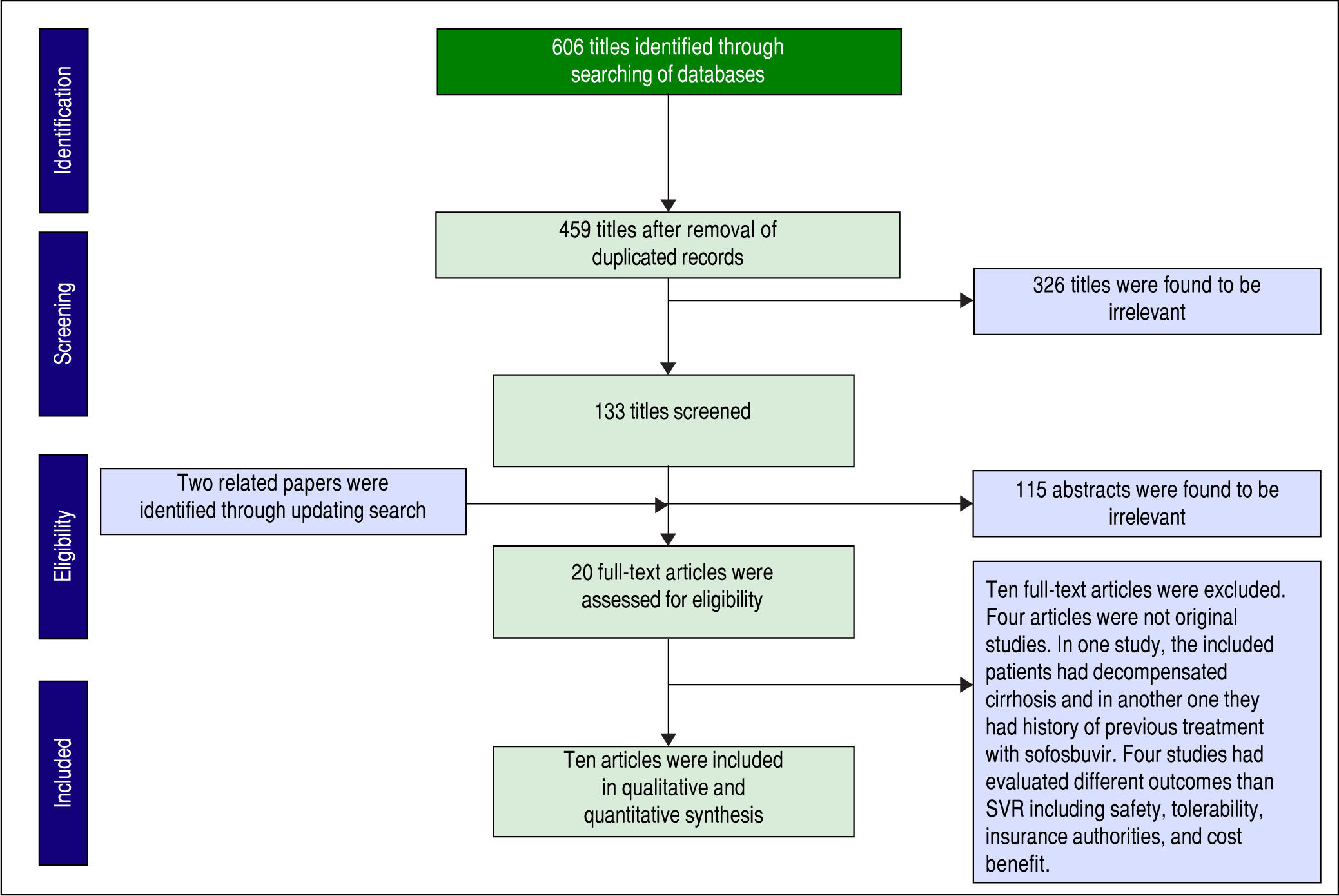
ResultsStudy screeningA total of 606 papers were identified via database searching. After removing duplications, 459 papers were remained for screening. Figure 1 shows the number of papers at each level of screening. Two other papers were also found by updating search. Twenty of full-text articles were assessed for eligibility and finally ten papers were included in our quantitative synthesis (meta-analysis).
Risk of bias assessmentConsidering table 1, all included studies were categorized as low risk (with taking a score of more than 7) and therefore no studies were excluded based on the quality assessment.
Risk of bias assessment for the included studies.
| First author (Reference) | Random sequence generation (Selection bias) | Allocation concealment (Selection bias) | Blinding of participant and personnel (Perfomance bias) | Blinding of outcome assessment (Detection bias) | Incomplete outcome data (Attrition) | Selective reporting (Reporting bias) | Co-interventions | Intention to treat analysis | Group similarity at baseline | Compliance | Timing of outcome assesments | Other biases | Score | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Afdhal, N.31 | ? | ? | + | + | - | - | - | - | - | - | - | - | 8 | Low |
| Afdhal, N. 7 | ? | ? | + | + | - | - | - | - | - | - | - | - | 8 | Low |
| Gane, E.J.13 | + | ? | + | + | - | - | - | - | - | - | - | - | 8 | Low |
| Lawitz, E.19 | - | - | + | + | - | - | - | + | - | - | - | - | 9 | Low |
| Kowdley, K.6 | ? | ? | + | + | - | - | - | - | - | - | - | - | 8 | Low |
| Bourliere, M.21 | - | - | - | - | - | - | - | + | - | - | - | - | 11 | Low |
| Mizokami, M.20 | - | - | + | + | - | - | - | - | - | - | - | - | 10 | Low |
| Stedman, C.A.M.14 | + | + | + | + | - | - | - | - | - | - | - | - | 8 | Low |
| Kohli, M.15 | ? | ? | + | + | - | - | - | - | - | - | - | - | 8 | Low |
| Chuang W.L.22 | ? | ? | + | + | - | - | - | - | - | - | - | - | 8 | Low |
Based on the goal of this study, we showed characteristics of each arms of the included papers in table 2 (combination of SOF/LDV) and table 3 (combination of SOF/ LDV/RBV).
Characteristics of the Included Studies for Combination of Sofosbuvir plus Ledipasvir.
| First Author (Reference) | History of Previous Treatment | Publication Year | Country | Sample Size | Mean Age (SD Or Range) | Male Gender (%) | Mean BMI (SD OR Range) | Treatment Duration (Wks) | Mean HCV RNA, Log IU/mL (SD) | Cirrhosis (%) | rs12979860 CC/CT+TTa | HCV Genotype 1a/1bb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Afdhal, N.31 | TE | 2014 | USA | 109 | 56 (24-67) | 74 (68) | 29 (19-47) | 12 | 6.5 (0.44) | 22 (20) | 0.1 | 3.73 |
| Afdhal, N.7 | TN | 2014 | USA | 214 | 52 (18-75) | 127 (59) | 27 (18-41) | 12 | 6.4 (0.69) | 34 (16) | 0.34 | 2.18 |
| Gane, E.J.13 | TE | 2014 | New Zealand | 10 | 61 (4.9) | 10 (100) | 31 (6.8) | 12 | 6.5 (0.6) | 10 (100) | 0.11 | 4 |
| Kowdley, K.6 | TN | 2014 | USA | 216 | 53 (20-71) | 128 (59) | 28 (19-45) | 12 | 6.4 (0.8) | 0 (0) | 0.35 | 3.9 |
| Lawitz, E.19 | TN | 2014 | USA | 19 | 46 (11.6) | 11 (58) | 28.1 (5.8) | 12 | 6.1 (0.8) | 0 (0) | 0.05 | 8.5 |
| Lawitz, E.19 | TE | 2014 | USA | 19 | 54 (6.6) | 15 (79) | 31.4 (4.7) | 12 | 6.3 (0.5) | 11 (58) | 0.11 | 18 |
| Kohli, A.15 | TN | 2015 | USA | 20 | 57 (8) | 14 (70) | 25 (4) | 12 | NA | up to 20% | 0.33 | 1.22 |
| Mizokami, M.20 | MIX | 2015 | Japan | 171 | 60 (9.2) | 69 (40) | 23.3 (3.6) | 12 | 6.6 (0.5) | 41 (24) | 1.011 | 0.042 |
| Chuang, W.L.22 | TN | 2016 | Taiwan | 42 | 54 (30-75) | 13 (31) | 24 (19-36) | 12 | 6.6 (0.68) | 5 (12) | 5 | 0.10 |
| Chuang, W.L.22 | TE | 2016 | Taiwan | 43 | 55 (33-7) | 23 (55) | 24 (20-30) | 12 | 6.6 (0.55) | 4 (9) | 1.26 | 0.16 |
| Afdhal, N.31 | TE | 2014 | USA | 109 | 56 (25-68) | 74 (68) | 28 (19-41) | 24 | 6.4 (0.57) | 22 (20) | 0.17 | 3.54 |
| Afdhal, N.7 | TN | 2014 | USA | 217 | 53 (22-80) | 139 (64) | 27 (18-48) | 24 | 6.3 (0.68) | 33 (15) | 0.31 | 2.14 |
| Bourliere, M.21 | TE | 2015 | France | 78 | 57 (10.7) | 56 (72) | 26.3 (4.2) | 24 | 6.5 (0.6) | 77 (100) | 0.08 | 1.85 |
Characteristics of the included studies for combination of sofosbuvir plus ledipasvir and ribavirin.
| First Author (Reference) | History of Previous Treatment | Publication Year | Country | Sample Size | Mean Age (SD Or Range) | Male Gender (%) | Mean BMI (SD OR Range) | Treatment Duration (Wks) | Mean HCV RNA, Log IU/mL (SD) | Cirrhosis (%) | rs12979860 CC/CT+TT* | HCV Genotype 1a/1b** |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Afdhal,N.31 | TE | 2014 | USA | 111 | 57 (27-75) | 71 (64) | 28 (19-45) | 12 | 6.4 (0.54) | 22 (20) | 0.11 | 3.82 |
| Afdhal,N.7 | TN | 2014 | USA | 217 | 52 (18-78) | 128 (59) | 27 (18-42) | 12 | 6.4 (0.64) | 33 (15) | 0.53 | 2.55 |
| Gane, E.J.13 | TN | 2014 | New Zealand | 25 | 45 (9.2) | 8 (32) | 25.2 (4.3) | 12 | 5.9 (0.9) | 0 (0) | 0.56 | 4 |
| Gane, E.J.13 | TE | 2014 | New Zealand | 9 | 50 (13) | 7 (78) | 25.6 (2.3) | 12 | 25.6 (2.3) | 0 (0) | 0 | 8 |
| Gane, E.J.13 | TE | 2014 | New Zealand | 9 | 57 (5.2) | 8 (89) | 27.3 (0.5) | 12 | 27.3 (0.5) | 9 (100) | 0.2 | 3.5 |
| Lawitz, E.19 | TE | 2014 | USA | 21 | 52 (9.8) | 14 (67) | 31.5 (7.3) | 12 | 6.2 (0.4) | 11 (52) | 0.05 | 3.2 |
| Mizokami, M.20 | MIX | 2015 | Japan | 170 | 59 (9.5) | 73 (43) | 23.3 (3.1) | 12 | 6.6 (0.5) | 35 (21) | 0.86 | 0.024 |
| Stedman, C.A.M.14 | MIX | 2015 | New Zealand | 14 | 54 (NA) | 12 (86) | 27 (34-20) | 12 | 6.5 (5.6-7.5) | 1 (7) | 0.4 | 2.5 |
| Bourliere, M.21 | TE | 2015 | France | 77 | 56 (7.4) | 58 (75) | 27.9 (5.5) | 12 | 6.5 (0.5) | 76 (98.7) | 0.05 | 1.71 |
| Afdhal, N.31 | TE | 2014 | USA | 111 | 55 (28-70) | 68 (61) | 29 (19-50) | 24 | 6.5 (0.60) | 22 (20) | 0.19 | 3.82 |
| Afdhal, N.7 | TN | 2014 | USA | 217 | 53 (24-77) | 119 (54) | 26 (18-48) | 24 | 6.3 (0.65) | 36 (17) | 0.5 | 2.01 |
We calculated pooled SVR12 for four HCV treatment regimens including 12 weeks of SOF/LDV (A), 24 weeks of SOF/LDV (B), 12 weeks of SOF/LDV/RBV (C) and 24 weeks of SOF/LDV/RBV (D). Summary of results of these meta-analyses has been shown in the table 4.
- •
Treatment regimen A (12 weeks of sofosbuvir plus ledipasvir). Ten arms in eight studies were found which evaluated regimen A (Table 2). There was significant heterogeneity between results of study arms (χ2 = 35.01, P < 0.001, I-squared = 74.3%) and thus pooled SVR12 for this regimen based on random-effect model was calculated as 95% (95%CI=92%-97%) (Figure 2A). Both Begg’s test (P = 0.074) and Egger’s linear regression test (P < 0.001) was significant and showed publication bias. We used trim and fil method to overcome this bias and according to this method Pooled SVR12 was calculated as 95% (95%CI = 93%-97%).
Figure 2.Pooled SVR12 for Sofosbuvir plus Ledipasvir Regimen by Treatment Duration and Addition of Ribavirin. A. Pooled SVR12 for 12 weeks of treatment with sofosbuvir plus ledipasvir. B. Pooled SVR12 for 24 weeks of treatment with sofosbuvir plus ledipasvir. C. Pooled SVR12 for 12 weeks of treatment with sofosbuvir plus ledipasvir and ribavirin. D. Pooled SVR12 for 24 weeks of treatment with sofosbuvir plus ledipasvir and ribavirin. TE: treatment experience. TN: treatment naïve.
(0.39MB). - •
Treatment regimen B (24 weeks of sofosbuvir plus ledipasvir). For treatment regimen B we found only three arms in three papers (Table 2). No significant heterogeneity was found regarding related studies to this regimen (χ2 = 1.92, P = 0.38, I-squared = 0%). Based on a fixed-effect model, pooled SVR12 for regimen B was 97% (95%CI = 95%-98%) (Figure 2B). Given the number of the included studies was small (n = 3) we did not run related tests for publication bias.
- •
Treatment regimen C (12 weeks of sofosbuvir plus ledipasvir and ribavirin). Nine study arms in seven papers were found for this type of regimen (Table 3). As no significant heterogeneity was found (χ2 = 8.64, P = 0.37, I-squared = 7.5%), fixed-effect model was used and pooled SVR12 calculated as 96% (95%CI = 94%-97%) (Figure 2C). There was publication bias based on both Begg’s (P = 0.009) and Egger’s linear regression (P = 0.001) tests. Based on the trim and fill method pooled SVR12 was calculated as 96% (95%CI = 94%-97%).
- •
Treatment regimen D (24 weeks of sofosbuvir plus ledipasvir and ribavirin). As table 3 shows, there were two study arms in two papers for this regimen. There was no significant heterogeneity regarding results of these two studies (χ2 = 0.77, P = 0.38, I-squared = 0%) and according to fixed-effect model, pooled SVR12 was calculated as 98% (95%CI = 97%-99%) (Figure 2D). Because of the small number of included studies (n = 2) we could not run the related tests for publication bias.
Summary of meta-analyses of the sustained virologic response rate for combination of sofosbuvir and ledipasvir.
| Regimen | Ribavirin Use | Treatment Duration (Wks) | SVR Rate (%) | 95%CI (%) |
|---|---|---|---|---|
| A | No | 12 | 95 | 93-97 |
| B | No | 24 | 97 | 95-98 |
| C | Yes | 12 | 96 | 94-97 |
| D | Yes | 24 | 98 | 97-99 |
- •
Cirrhosis. In most study arms for these four mentioned treatment regimens, the whole patients were cirrhotic or non-cirrhotic and therefore we could not run a meta-analysis of odds ratio (OR). However, we combined data of each arm related to each treatment regimen and finally calculated the OR for effect of cirrhosis on SVR12 using Peto method. Considering Figure 3A, only in treatment regimen A, cirrhosis had a significant effect on SVR12 (OR = 0.21, 95%CI = 0.07-0.66).
- •
Previous history of treatment. We evaluated the effect of previous history of treatment on SVR12. Figure 3B shows combined data related to each treatment regimen and ORs (according to Peto method) related to effect of previous history of treatment. Based on this analysis, previous history of treatment had no significant effect on SVR12 in all regimens.
- •
NS5A resistance-associated substitutions. Data regarding NS5A RASs were extracted from the included studies which evaluated RASs at baseline. Because data about this issue were not available for evaluation of each regimen (A, B, C and D), inevitably we investigated the effect of the RASs on the main regimen SOF/ LDV with or without RBV for 12 or 24 weeks. Data of three papers were not included in this analysis; Gane, et al.13 because of adding another medication (GS-9669) to SOF/LDV regimen in two study arms, Stedman, et al.14 because of unavailable data of evaluation of RASs on SVR and Kohli, et al.15 because of reporting inadequate data about number of patients with SVR and NS5A RASs. Moreover, Kowdley and coworkers6 evaluated the effect of SOF/LDV for 8 weeks in one arm of their study and as we could not separate reported data about NS5A RASs and SVR from this arm, so we extracted all the data from this study and included them in this meta-analysis. According to Figure 4, NS5A RASs had a significant reduction effect on the SVR in SOF/LDV regimen (OR = 0.31, 95% CI = 0.2-0.5). There was no heterogeneity across results of studies regarding detection of NS5A RASs at baseline and treatment response. Also, based on Begg’s (P = 0.65) and Egger’s (P = 0.67) tests, no publication bias was found.
The current meta-analysis showed the high efficacy of SOF/LDV combination therapy in patients with chronic hepatitis C genotype 1 infection. The previous standard of care for treatment of HCV genotype 1 infection was PegIFN and RBV with less than 60% SVR rate in patients with HCV genotype 1 infection and the treatment accompanied by many side-effects.16
The recognition of key proteins in the HCV replication cycle provided the opportunity to target these proteins and inhibit the production of virions.17 HCV treatment regimens has had a long story from IFN-based to IFN-free regimens. This provided a treatment regimen with high SVR rate, short treatment duration and small number of adverse events. The goal of having an IFN-free treatment regimen for HCV genotype 1 infection was achieved with introduction and approval of SOF/LDV regimen in 2014.18
The SOF/LDV can be used with or without RBV and in different treatment duration (12 or 24 weeks).7 Some important factors can be considered for choosing appropriate regimen such as existence of cirrhosis and RBV contraindication. However, the cost of this regimen is another considerable factor for choosing the duration of treatment. Interestingly, in the current study, it has been shown that SVR in all regimens of 12 or 24 weeks with or without RBV were equal and more than 95%, including; 95% for 12 weeks of treatment with SOF/LDV, 97% for 24 weeks of treatment with SOF/LDV, 96% for 12 weeks of treatment with SOF/LDV/RBV and 98% for 24 weeks of treatment with SOF/LDV/RBV. It is true that addition of RBV to SOF/LDV or prolongation of SOF/LDV to 24 weeks can result in a slight increase in SVR12 from 95% to 96% however, in the current study, it was observed that cirrhosis (Child-Pugh A) can impact (OR = 0.21) the SVR12 just in regimen of 12 weeks of treatment with SOF/LDV. As a result, we recommend to treat cirrhotic patients (Child-Pugh A) with SOF/LDV/RBV for 12 weeks or with SOF/LDV for 24 weeks based on the RBV contraindication and the price issue while it seems treatment of non-cirrhotic patients with SOF/LDV for 12 weeks is acceptable. In this meta-analysis, the impact of previous history of treatment (other than SOF-based regimens) on treatment success in none of the four regimens of SOF/LDV was observed. As a result, selection of the treatment duration or adding RBV to SOF/LDV regimen based on the history of previous treatment with PegIFN/RBV or PI-containing regimens is not recommended.
In this study, we collected the data for assessment of the impact of baseline NS5A RASs on treatment success however in none of these studies the data for resistance assessment was stratified regarding the treatment regimen. As a result, the data for all arms of each study was included in this meta-analysis. This meta-analysis found the baseline RASs of NS5A gene of HCV as a parameter impacted treatment response rate. It is worth to note, that most of patients with treatment failure harbored NS5A RASs at the time of treatment failure which shows the major role of NS5A RASs in treatment failure of patients treated with LDV.6,7,19-22 However, clinical utility for assessment of NS5A RASs before starting the treatment is not defined yet maybe because of the high treatment response rate to SOF/LDV and very low specificity of assessment of NS5A RASs for prediction of treatment failure.
The introduction of other DAAs and IFN-free regimens and their approval for treatment of patients with HCV genotype 1 infection was continued after approval of SOF/LDV. In December 2014, combination of Ombitas-vir/Paritaprevir-r/Dasabuvir (3 direct acting antiviral; 3D) for HCV genotype 1 infection with more than 95% efficacy was approved.23 In January 2016, the combination therapy with Grazoprevir/Elbasvir (GZR/EBR) with about 95% SVR rate was approved.24 Given, SOF has renal metabolism, this antiviral agent cannot be administered in chronic kidney disease (creatinine clearance < 30 mL/min/1.73 m2) and the 3D and GZR/EBR are the alternatives for treatment of patients with chronic kidney disease and HCV genotype 1 infection.25,26 Furthermore, other combinations of DAAs such as SOF/Daclatasvir (27) and SOF/ Velpatasvir28 have been available as pan-genotypic treatment regimens.
In conclusion, the treatment of HCV genotype 1 with SOF/LDV combination results in high (≥ 95%) treatment response. The decision to add RBV to SOF/LDV and/or prolongation of SOF/LDV can be made based on presence of cirrhosis, contraindication of RBV and the cost issue. More identification of HCV-infected people and providing more access to treatment for them can help for HCV elimination.29 The next goal in the field of HCV treatment is the development of new HCV therapeutic regimens without RBV with a promising 100% treatment response and no drug-drug reaction.30
AcknowledgementsAuthors’ Contribution: Study concept and design: Mohammad Saeid Rezaee-Zavareh, Bita Behnava, Seyed Moayed Alavian and Heidar Sharafi; acquisition of data: Mohammad Saeid Rezaee-Zavareh and Khashayar Hesa-mizadeh; analysis and interpretation of data: Mohammad Saeid Rezaee-Zavareh, Mohammad Gholami-Fesharaki and Heidar Sharafi; drafting of the manuscript: Mohammad Saeid Rezaee-Zavareh, Khashayar Hesamizadeh, Mohammad Gholami-Fesharaki and Heidar Sharafi; critical revision of the manuscript for important intellectual content: Bita Behnava and Seyed Moayed Alavian.
Authors’ Declaration Of Personal InterestsNone declared.
Declaration of Funding InterestsNone declared.