HBV and HCV reactivation has been widely reported in patients undergoing immunosuppressive therapy for oncohaematological diseases. We aimed to evaluate the HBV and HCV reactivation events in patients with non-Hodgkin lymphoma (NHL) or Hodgkin lym-phoma (HL) underwent cytotoxic chemotherapy containing or not rituximab. This is a retrospective observational study, including all patients with NHL and HL attending an Italian tertiary referral hospital, the University of Naples “Federico II”. A total of 322 patients were enrolled. We evaluated serum HBV and HCV markers. A total of 47 (38%) patients with occult HBV infection were enrolled. Seven/47 were treated with therapeutic cytotoxic schedule containing rituximab. Of them, 6/7 received prophylaxis with lamivudine. HBV reactivation was observed in two patients treated with rituximab. A reactivation was observed in the only patient (HBcAb+/HB-sAb+) not receiving lamivudine prophylaxis, and the other one was observed in 1 patient with isolated HBcAb positivity during lami-vudine prophylaxis. Moreover, 8 patients with HCV-Ab positivity were enrolled. No viral reactivation was observed in these patients. In conclusion, patients with occult HBV infection receiving chemotherapy containing rituximab for lymphoma without antiviral prophylaxis are at risk of viral reactivation. On the contrary, there is no risk of reactivation in patients undergoing rituximab-free schedule. Our findings suggest that there is also very low risk of HCV reactivation. This preliminary report underlines the concept that HBV reactivation is strongly related to the type of immunosuppressive therapy administered and that antiviral prophylaxis needs to be tailored.
Hepatitis B virus (HBV) infection is a global health is-sue1 and it is a common cause of liver disease, affecting more than 240 million people worldwide.2 HBV carriers are traditionally identified by detection of the virus surface antigen (HBsAg) in their blood. However, the possible persistence of HBV genomes in HBsAg-negative patients has been definitively proven. This particular form of viral persistence –commonly termed “occult” HBV infection (OBI)– is defined as the presence of HBV-DNA in the serum and/or in the liver of individuals testing negative for HBsAg and positive for antibodies directed against the HBV-core gene products (HBcAb) by currently available assays.3-5 What is well known about this silent infection is that it can represent a life-threatening risk factor if the patient becomes immunocompromised.6-16 HBV reactivation in patients with haematological malignancies undergoing chemo-immunotherapy is a frequent and severe complication.16-22 Reactivation occurs in HBsAg-positive patients and in patients with OBI receiving immuno-suppressive chemotherapy, with a rate of mortality due to acute liver failure ranging between 45% and 85%.12,17,23,24 This critical issue has been prevalently analyzed in patients with lymphoma and the majority of therapeutic randomized controlled trials has focused on it,13,25,26 especially in case of Rituximab plus-steroid combination chemotherapy.27-32 The incidence of HBV reactivation in patients with lymphoma and OBI after rituximab-based therapy ranges from as low as 1.5% to 23.8%.28,33-36 This topic has received growing scientific interest and several studies reported the protective effect of HBV antiviral drugs on reactivation during immunosuppressive therapy. Identifying the patients at risk is mandatory in this clinical setting and prophylaxis with antiviral drugs or close monitoring may reduce and/or eliminate the hepatitis flare-up due to HBV reactivation and its serious clinical consequences.
The prevalence of hepatitis C virus (HCV) infection is reported to be higher in patients with lymphoma (15%) than general population (1.5%), particularly in geographical areas with high incidence of HCV infection.37 However, data on the consequence of chemotherapy on the course of HCV infection in patients with lymphoma have been controversial.38-41 Little is known on the changes in HCV replication and associated ALT-flares during chemotherapy. This suggests that chemotherapy potentially induces HCV replication and viral load decreases toward baseline after completion of immunochemotherapy. On the other hand, HCV reactivation has been reported to be associated with liver damage or hepatic dysfunction, but fulminant hepatitis due to HCV reactivation is a rare com-plication.40
This single-center retrospective study was designed to evaluate the HBV and HCV reactivation events in patients with non-Hodgkin lymphoma (NHL) or Hodgkin lym-phoma (HL) underwent cytotoxic chemotherapy containing or not rituximab. A secondary aim was to assess the frequency of HBV and HCV serological markers in a series of patients with NHL or HL.
Material and MethodsSetting, design and patientsThis is a retrospective, observational study carried out at the Haematology Unit of the University of Naples “Fe-derico II”, a tertiary referral centre in Southern Italy. The target population consisted of adult patients living in this area, which is considered endemic for HBV and HCV in-fection.4,42
This study was independently designed by the authors, conducted in compliance with the 1975 Declaration of Helsinki and approved by the Ethic Committee of the University of Naples Federico II.
The medical records of the patients admitted to the Haematology Unit, in collaboration with the Gastroenter-ology Unit, who received chemotherapy regimens for lymphoma from January 2006 to June 2014, were retrospectively reviewed.
To be eligible in the study, patients needed to meet the following criteria:
- •
Age ≥ 18 years.
- •
Histology-proven Hodgkin lymphoma or non-Hodg-kin lymphoma.
- •
Classification of lymphoma according to Ann-Arbor/ Cotswolds staging system.
- •
Completion of all treatment scheduled for haemato-logical remission induction.
Patients with HIV or HDV co-infection, or lost to follow-up, were excluded. Before starting chemo-im-munosuppressive therapy, all patients had been routinely tested for serology of HBV and HCV. For patients with HBV or HCV infection, a scheduled monitoring had been applied: transaminases every month, complete liver function test (bilirubin, INR, g-Glutamyl-Trans-ferase, alkaline phosphatase, albumin) every 3 months and HBV/HCV serological status (HBsAg and HBV-DNA for HBV infection, HCV-RNA for HCV infection) every 3 months from the start of therapy, until the last follow-up visit.
Records for 322 outpatients were reviewed in relation to the markers of occult or active HBV infection and HCV status. Among these, 110 patients showed at least 1 positive marker of HBV or HCV infection.
Definition of HBV and HCV infectionHBV chronic infection was defined according to EASL guidelines29 as patients positive for HBsAg, independently from the HBeAg/HBeAb positivity and the HBV-DNA levels. “Occult” HBV infection represents a particular clinical entity that is characterized by the persistence of HBV-DNA in the liver tissue, without the evidence of overt HBV infection in individuals who are HBsAg-nega-tive and HBcAb-positive either with or without serum HBV-DNA positivity.43 The difficulty in identifying HBV-DNA in liver biopsy (frequently not justified in patients without clinical signs of hepatitis) and the rarity of detectable serum viremia, even with sensitive techniques, lead to consider all HBsAg negative-HBcAb-positive patients (HBV-DNA negative, with or without HBsAb-pos-itivity) as potential OBI.43
HCV infection was defined as patients positive for HCV-Ab; in particular, the infection was considered active if HCV-RNA was positive, and resolved if HCV-RNA was negative [with no risk of viral reactivation].44
Definition of HBV and HCV reactivationIn HBV-positive patients, the following biochemical events were considered significant for a viral reactivation:
- •
In HBsAg-positive patients (active or inactive carri ers), the increase of at least one logarithm of HBV- DNA, with or without the concomitant increase of transaminases;
- •
In potential OBI (HBcAb positive and/or HBsAb-pos-itive patients), the re-emergence of HBsAg or the appearance or increase of at least one logarithm of HBV-DNA.
Reactivation of HCV was defined as a significant increase of HCV-RNA (at least one logarithm), regardless of a concomitant increase of transaminases. In HCV-Ab positive but HCV-RNA negative patients (resolved HCV infection), there is no risk of reactivation.
Serological profileHBsAg, HBcAb and HBsAb were determined by conventional commercial assay kits (Abbott AxSYM AUSAB, Germany; HBsAg EIA, Abbott, North Chicago, IL). HCV-Ab and HDV-Ab were determined by commercial enzyme linked immunosorbent assay III, Abbot Laboratories Chicago. All HBcAb-positive samples were assayed for serum HBV-DNA by a commercial qualitative target amplification method (Cobas Ampliscreen, Roche Molecular Systems, Branchburg, New Jersey, USA). In order to achieve the highest sensitivity allowed by this method (20 IU/mL), testing was performed on each individual sample without pooling and increasing the volume for extraction (500 μL). The specimens that resulted positive were further tested by a quantitative method (Cobas Amplicor HBV Monitor, Roche Molecular Systems, Branchburg, NJ) to determine the viral load.
All HCV-Ab positive samples were assayed for serum HCV-RNA by a qualitative method (Cobas Ampliscreen, Roche Molecular Systems, Branchburg, NJ). In order to achieve the highest sensitivity allowed by this method (15 IU/mL), testing was performed on each individual sample-without pooling-and increasing the volume for extraction (500 μL). The specimens that resulted positive were further tested by quantitative method (Light Cycler Instrument, Roche Molecular Biochemicals, Mannheim, Germany) to determine the viral load.
Schedules of chemoimmunotherapiesR-CHOP regimen consisted of a 3-week course of the schedule of rituximab 375 mg/m2 i.v. day 1, cyclophos-phamide 750 mg/m2 i.v. day 1, doxorubicin 50 mg/m2 i.v. day 1, vincristine 1.4 mg/m2 i.v. day 1, and prednisone 100 mg os daily. Patients with follicular lymphoma or Diffuse Large B-Cell Lymphoma (DLBCL) received six courses of R-CHOP. From 2010, patients with follicular lymphoma responding to R-CHOP underwent maintenance with rituximab (375 mg/m2 i.v., every 8 weeks) for 2 years.
CHOP regimen consisted of a 3-week course of the schedule of cyclophosphamide 750 mg/m2 i.v. day 1, doxo-rubicin 50 mg/m2 i.v. day 1, vincristine 1.4 mg/m2 i.v. day 1, and prednisone 100 mg os daily. Patients with T-cell NHL received six courses of CHOP.
Patients with aggressive NHL and bulky disease at diagnosis responding to R-CHOP o CHOP underwent irradiation (32 Gy) of residual masses at the initial sites of bulky disease.
ABVD regimen consisted of a 4-week course of the schedule of doxorubicin 25 mg/m2 i.v. day 1 and 15, bleo-mycin 10 mg/m2 i.v. day 1 and 15, vinblastine 6mg/m2 i.v. day 1 and 15, and dacarbazine 375 mg/m2 i.v. day 1 and 15. Patients with HL and advanced disease (stages II-B, III-IV) received six courses of ABVD followed by irradiation of residual masses at the initial sites of bulky disease.
Schedule of lamivudine prophylaxisAntiviral prophylaxis was started in HBsAg negative – HBcAb positive patients, with or without HBsAb positiv-ity, treated with rituximab schedules and it consisted of an oral administration of lamivudine (100 mg/day). The prophylaxis started at least 4 weeks before starting chemotherapy, continued during Rituximab administration and for an additional 12 months after the end of chemotherapy.
Statistical analysisDemographical, clinical, biochemical, histological, vi-rological and therapeutic data were collected from medical records in case report form. Baseline characteristics were expressed as median and range for continuous and not normally distributed data, as mean and standard deviation (SD) for normally distributed data and as percentage for categorical data.
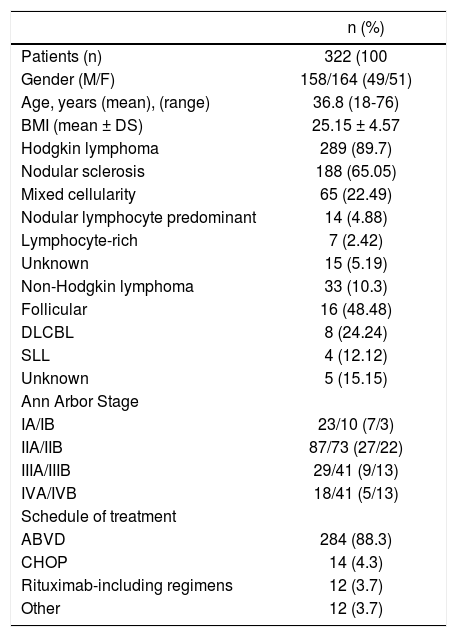
ResultsThree hundred and twenty-two patients fulfilling inclusion criteria were enrolled in the study. The characteristics of this population are illustrated in table 1.
Characteristics of patients with lymphoma at baseline.
| n (%) | |
|---|---|
| Patients (n) | 322 (100 |
| Gender (M/F) | 158/164 (49/51) |
| Age, years (mean), (range) | 36.8 (18-76) |
| BMI (mean ± DS) | 25.15 ± 4.57 |
| Hodgkin lymphoma | 289 (89.7) |
| Nodular sclerosis | 188 (65.05) |
| Mixed cellularity | 65 (22.49) |
| Nodular lymphocyte predominant | 14 (4.88) |
| Lymphocyte-rich | 7 (2.42) |
| Unknown | 15 (5.19) |
| Non-Hodgkin lymphoma | 33 (10.3) |
| Follicular | 16 (48.48) |
| DLCBL | 8 (24.24) |
| SLL | 4 (12.12) |
| Unknown | 5 (15.15) |
| Ann Arbor Stage | |
| IA/IB | 23/10 (7/3) |
| IIA/IIB | 87/73 (27/22) |
| IIIA/IIIB | 29/41 (9/13) |
| IVA/IVB | 18/41 (5/13) |
| Schedule of treatment | |
| ABVD | 284 (88.3) |
| CHOP | 14 (4.3) |
| Rituximab-including regimens | 12 (3.7) |
| Other | 12 (3.7) |
All patients had malignant lymphoma; in particular 33 had NHL and 289 had HL. Out of 322 patients, 110 showed at least 1 positive marker of HBV or HCV infection. No differences in terms of sex, age, lymphoma subtype, chemotherapy regimens were observed between patients regardless of HBV or HCV infection. Rituximab regimen was administered in 12 patients, while 310 patients were treated with a Rituximab-free schedule (ABVD or CHOP). None was treated by autologous peripheral blood stem cell transplantation or allogenic stem cell transplantation.
HBV infectionIn total, 102/322 patients (31.6%) showed at least 1 marker of HBV infection: 4/322 patients (1.24%) were HBsAg-positive, 34/322 patients (10.5%) were isolated HBcAb-positive, and 13/224 patients (4%) were HBcAb and HBsAb positive. Fifty-one/322 patients (15.8%) were HBsAb-positive only, reflecting a vaccination status. None of these patients showed viral reactivation.
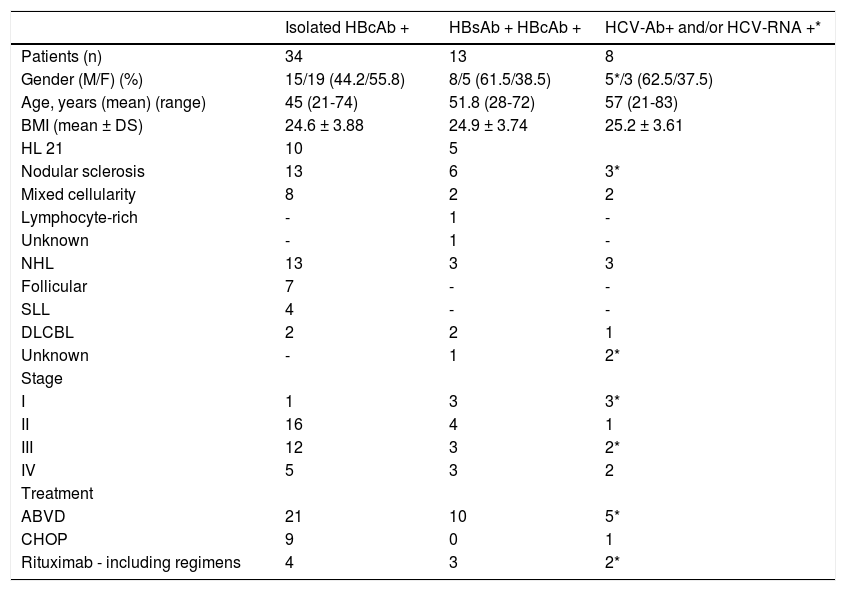
The main features of the 47 patients with potential OBI infection are reported in table 2. Seventeen patients were affected by NHL and 34 by HL. None of these showed signs of cirrhosis. At the baseline all patients had normal aminotransferase levels. The schedules of cytotoxic treatment are reported in table 2. Forty-four patients were treated with Rituximab-free schedule (ABVD or CHOP), while 7 patients were treated with rituximab regimens. Due to the extended retrospective nature of the present study and the low number on NHL, the prevalence of rituximab containing protocols is slightly lower than that observed in the current clinical practice. No statistical significant differences were found between patients treated with and without rituximab, except for the different rates of aggressive lymphomas that were more frequently treated with rituximab. All HBsAg-positive patients were treated with antiviral drugs (the 3 patients with inactive hepatitis were treated with lamivudine and the remaining patient with active hepatitis continued the pre-exisisting antiviral therapy with tenofovir) before starting Rituxi-mab-free chemotherapy. Of the 47 HBcAb-positive patients, regardless of HBsAb-positivity, 7 were treated with chemotherapy containing rituximab. Of these 7 patients, 6 (4/4 isolated HBcAb and 2/3 HBcAb-positive/HBsAb-positive) were treated with lamivudine as prophylaxis therapy, that was prolonged for 12 months after immuno-suppressive therapy. The characteristics of these patients are reported in table 3.
Characteristics of patients with lympho ma and HBV/HCV infection at baseline.
| Isolated HBcAb + | HBsAb + HBcAb + | HCV-Ab+ and/or HCV-RNA +* | |
|---|---|---|---|
| Patients (n) | 34 | 13 | 8 |
| Gender (M/F) (%) | 15/19 (44.2/55.8) | 8/5 (61.5/38.5) | 5*/3 (62.5/37.5) |
| Age, years (mean) (range) | 45 (21-74) | 51.8 (28-72) | 57 (21-83) |
| BMI (mean ± DS) | 24.6 ± 3.88 | 24.9 ± 3.74 | 25.2 ± 3.61 |
| HL 21 | 10 | 5 | |
| Nodular sclerosis | 13 | 6 | 3* |
| Mixed cellularity | 8 | 2 | 2 |
| Lymphocyte-rich | - | 1 | - |
| Unknown | - | 1 | - |
| NHL | 13 | 3 | 3 |
| Follicular | 7 | - | - |
| SLL | 4 | - | - |
| DLCBL | 2 | 2 | 1 |
| Unknown | - | 1 | 2* |
| Stage | |||
| I | 1 | 3 | 3* |
| II | 16 | 4 | 1 |
| III | 12 | 3 | 2* |
| IV | 5 | 3 | 2 |
| Treatment | |||
| ABVD | 21 | 10 | 5* |
| CHOP | 9 | 0 | 1 |
| Rituximab - including regimens | 4 | 3 | 2* |
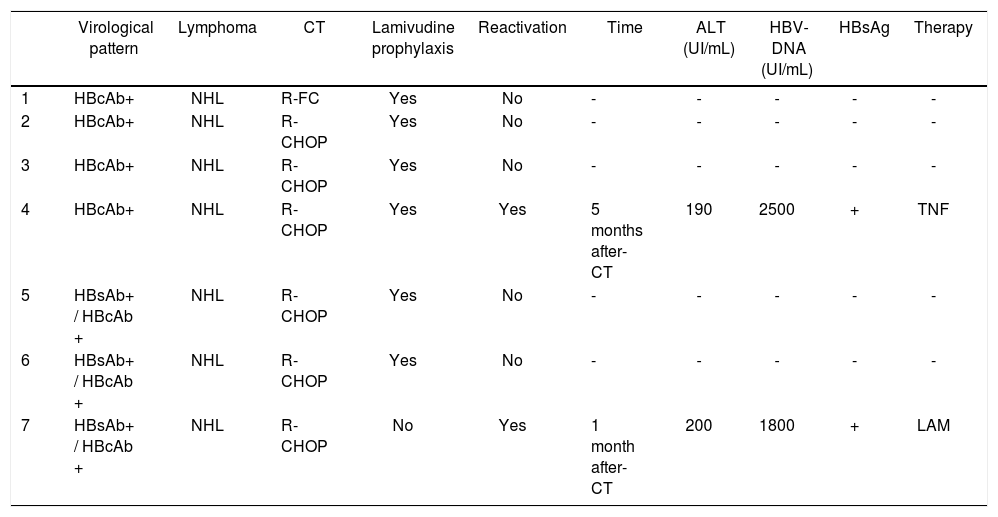
Characteristics of patients with lymphoma and HBV infection treated with rituximab - including regimens.
| Virological pattern | Lymphoma | CT | Lamivudine prophylaxis | Reactivation | Time | ALT (UI/mL) | HBV-DNA (UI/mL) | HBsAg | Therapy | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | HBcAb+ | NHL | R-FC | Yes | No | - | - | - | - | - |
| 2 | HBcAb+ | NHL | R-CHOP | Yes | No | - | - | - | - | - |
| 3 | HBcAb+ | NHL | R-CHOP | Yes | No | - | - | - | - | - |
| 4 | HBcAb+ | NHL | R-CHOP | Yes | Yes | 5 months after- CT | 190 | 2500 | + | TNF |
| 5 | HBsAb+ / HBcAb + | NHL | R-CHOP | Yes | No | - | - | - | - | - |
| 6 | HBsAb+ / HBcAb + | NHL | R-CHOP | Yes | No | - | - | - | - | - |
| 7 | HBsAb+ / HBcAb + | NHL | R-CHOP | No | Yes | 1 month after- CT | 200 | 1800 | + | LAM |
HBV reactivation was observed in two patients. The major characteristics of these patients are reported in table 3.
The first reactivation was observed in a 52-year old woman with NHL, II stage. The virological pattern at the diagnosis of NHL was: HBsAg-negative, HBcAb-positive, HBsAb-positive, HBV-DNA negative. She was treated with R-CHOP schedule from March 2011 to August 2012 (71 weeks of treatment). She did not receive lamivudine prophylaxis before starting chemotherapy. The reactivation occurred 1 month after the end of chemotherapy. The patient showed increase of transaminases and reappearance of HBsAg and HBV-DNA. The patient received lamivudine therapy immediately. She recovered within two months.
The second reactivation was observed in a 61-year old woman with NHL, IV stage. The virological pattern at the diagnosis of NHL was: HBsAg negative, HBcAb positive, HBsAb negative, HBV-DNA negative. She was treated with R-CHOP schedule from January 2013 to June 2013 (26 weeks of treatment). She received lamivudine prophylaxis before starting chemotherapy, maintaining HBV-DNA negativity for all the chemotherapy. The reactivation occurred 5 months after the end of chemotherapy, during lamivudine therapy. The patient showed an increase of transaminases and reappearance of HBsAg and HBV-DNA. She was adherent to therapy and no viral resistance was documented. She immediately stopped lamivudine therapy and started tenofovir. The patient recovered within one month.
Resuming, the Relative Risk for HBV reactivation in patients treated with rituximab is 6.8 times higher than in patients treated without rituximab, despite the prophylaxis with lamivudine.
None of the other 44 patients treated with cytotoxic chemotherapy without rituximab (ABVD/CHOP, 32/8) and without receiving prophylaxis with lamivudine, revealed HBV reactivation.
HCV infectionIn total, 8/322 (2.5%) patients had a positive serology for HCV infection, i.e HCV-Ab positivity (5 male and 3 female, mean age 57yrs). The main features of the 8 subjects with HCV-Ab positivity are reported in table 2. In particular, 6/8 were HCV-RNA negative and 2/8 were positive. The prevalence of active HCV infection (i.e. with HCV-RNA positivity) was 0.6%. Three patients were affected by NHL and 5 by HL. None of these showed signs of cirrhosis. All HCV patients had baseline normal ami-notransferases levels. The schedules of treatment are reported in table 2. Six patients were treated with cytotoxic chemotherapy without rituximab, while 2 patients were treated with chemotherapy containing rituximab. Serum liver functional tests in all cases showed no significant changes in transaminases levels and for the 2 patients with active infection no changes in viral load, from baseline to the last follow-up visit (12 months after the end of chemotherapy).
DiscussionPrevalence of hepatitis infectionIn literature is reported a higher prevalence of HBV and HCV infection in patients with lymphoma. Moreover, a large body of evidence sustains that HBV and HCV infections are risk factors for lymphoma development4,37,45 and this association could also be underestimated because of the possibility of occult HBV infection.46-48
The total prevalence of HBV and HCV markers in our population of HL and NHL is 15.8% and 2.5%, respectively, confirming the strong association between these viruses and lymphoma, but also the higher prevalence of these infections in South Italy. Indeed, the prevalence of OBI is higher than the one reported in the general population, but it has been already described in patients with lymphoma.46-48 On the other hand, our findings are in agreement with a recent Korean study that reports a prevalence of potential occult HBV infection and resolved HCV infection in patients with lymphoma of 12.4% and 2.8%, respectively.49
Concerning the HBV coverage rate, in this survey the vaccination rate in the population ages in the program of universal vaccination (established in Italy in 1991) is much lower than reported in literature (38.7% vs. 63%).50,51 South Italy was considered a highly endemic country for HBV before the implementation of universal vaccination, and the lower coverage rate was probably due to early leaving of school by children generally belonging to needy classes and then at potentially higher risk of acquiring HBV infection.42
Viral reactivationCurrent evidence supports that HBsAg-positive patients should receive prophylactic antiviral therapy prior to initiating immunosuppressive therapy in all clinical settings. As a matter of fact, none of the enrolled HBsAg-pos-itive patients experienced episodes of reactivation, since all of them were treated with antiviral therapy. In haema-tological setting, HBsAg-negative/HBcAb-positive patients receiving chemotherapy containing rituximab for lymphoma, in absence of antiviral prophylaxis, are at high risk of HBV reactivation. On the other hand, the risk of reactivation in patients undergoing rituximab-free schedules seems to be very low.52,53
The risk of reactivation for the category of potential OBI is controversial and seems to be related to the clinical setting and the type of immunosuppressive therapy. There are several reported cases of HBV reactivation in HBcAb-positive patients, regardless of concomitant HB-sAb-positivity, who have undergone bone marrow transplantation or cytotoxic chemotherapy for lymphoma.10,54-58 In these patients, the use of intense immunosuppression, like monoclonal antibodies anti-lymphocyte B and T (anti-CD20), is particularly considered as risk factor.32 The general agreement of European Association for the Study of the Liver (EASL) about the management of this kind of patients is well known.43 Nonetheless, there is no consensus regarding the optimal management strategy for occult HBV infection among the different guidelines.43,59-61 In particular, even if a very careful monitoring is suggested, different approaches are reported, especially regarding the type of anti-neoplastic therapy. Because of these findings, EASL and AGA (American gastroenterological association) suggested prophylaxis with lamivudine in all HBcAb-positive/HBsAg-negative patients who are assigned to highly immunosuppressive treatments for hae-matological malignancies (this approach should be justified by the low toxicity and low cost of orally administered lamivudine),43,59 while ASCO (American Society of Clinical Oncology), APASL (Asian pacific association for the study of the liver) and AASLD (American association for the study of the liver) consider that in occult HBV infection reactivation is infrequent, so these patients should be monitored and antiviral therapy initiated when HBV reactivation occurs.60-62 Nevertheless, in our HB-cAb-positive cohort, HBV reactivation occurred in patients treated with rituximab and in whom it was administered lamivudine prophylaxis. Until now antiviral prophylaxis was performed with lamivudine, even though long-term treatment involves a risk of developing drug resistance with hepatitis flare-ups and risk of acute liver failure and/or delayed of scheduled chemotherapy. For these reasons, entecavir or tenofovir may represent a better alternative to lamivudine as they have better efficacy, tolera-bility, and high genetic barriers to resistance.26 Not enough data are available about this topic, and further studies are needed.
Masarone, et al, in a recent study, reports 10 cases of HBV reactivation in OBI patients with NHL, treated with and without rituximab and without antiviral prophylaxis, with a reactivation rate of 10.4%.10 The reactivation rate observed in our population in HBsAg-negative patients is lower. Probably, our result can be explained by the contemporary administration of lamivudine prophylaxis to almost all patients, differently from the cited study.10
With regard to patients with HCV infection, the use of immunosuppressive therapy appears to determine lower frequency and severity of viral reactivation than in HBV infection.38 This finding is confirmed in our 2 patients with active HCV infection undergoing chemotherapy, containing or not rituximab, in which no episodes of viral reactivation was observed. Concerning this topic, in literature are reported few cases of HCV reactivation during treatment for lymphoma.40,41 In particular, these studies described an increase in HCV-RNA of at least 1 logarithm during rituximab-based chemotherapy, and ALT-flare after discontinuation, life-threatening in one with cirrhosis.40
Although this research has reached its aims, there were some unavoidable limitations. First of all, the small sample size of the studied groups, that makes a powerful statistical analysis difficult and a generalizability of the obtained results. A second potential limit of this study is that the majority of our population consists of patients with HL (89.7%), because the enrollment was carried out in the referral haematological centre for the management of this kind of lymphoma, for which milder haematologi-cal therapies are used (standard protocols without monoclonal antibodies) differently from the population described by Masarone, et al, that constists of only patients with NHL.10 However, this limit provides information on a population less studied until now. Another limitation relies on its retrospective nature that cannot exclude unintended and confounding biases, and that caused an incompleteness of data regarding HBV and HCV genotyp-ing, or testing for mutations conferring resistance to lami-vudine, and so on.
In conclusion, screening for HBV and HCV is now recommended for all patients with lymphoma before starting chemotherapy. All patients with HL and NHL should be tested for HBV(including HBsAg, HBcAb, HBsAb and HBV-DNA if needed) and HCV (including HCV-Ab and HCV-RNA if HCV-Ab positive) markers to assess the infection or vaccination status. HBV vaccination is mandatory in all seronegative patients.
The HBsAg-negative/HBcAb-positive patients (i.e., with a potential OBI) receiving chemotherapy containing rituximab for lymphoma without antiviral prophylaxis are at risk of HBV reactivation. On the contrary, our findings suggest that there is no risk of reactivation in patients undergoing rituximab-free-schedules, in particular with ABVD and CHOP regimens.
Our study underlines the concept that HBV reactivation is strongly related to the type of immunosuppres-sive therapy administered and, as a consequence, antiviral prophylaxis needs to be tailored. Finally, we confirm the need of at least a strict surveillance of these patients in order to prevent HBV reactivations, which is a life-threatening condition if not rapidly recognized and treated.
Abbreviations- •
HBV: hepatitis B virus.
- •
HBsAg: virus surface antigen.
- •
OBI: “occult” HBV infection.
- •
HBcAb: antibodies directed against the HBV core protein.
- •
NHL: non-Hodgkin lymphoma.
- •
HL: Hodgkin lymphoma.
- •
DLBCL: diffuse Large B-Cell Lymphoma.
- •
SD: standard deviation.
- •
EASL: European Association for the Study of the Liver.
- •
Study concept and design: Morisco F, Caporaso N, Picardi M, Pane F.
- •
Acquisition of data: Guarino M, Pugliese N, Rea M, Vitiello A, Cossiga V.
- •
Analysis and interpretation of data: Guarino M.
- •
Drafting of the manuscript: Guarino M.
- •
Critical revision of the manuscript for important intellectual content: Morisco F, Picardi M.
- •
Statistical analysis: Guarino M.
- •
Study supervision: Morisco F.
This study was supported by Gilead Fellowship Program 2012.
Conflict of Interest Statement:The authors declare that they have none study sponsors and none conflict of interest/financial disclosures in relation to this study.