Background: Non-alcoholic fatty liver disease (NAFLD) is common in obese and diabetics. Serine protease inhibitor Kazal-1 (SPINK-1) protein is highly expressed in the liver and adipose tissue of diabetic and obese suggesting its role in NAFLD. SPINK-1 also behaves as an acute phase reactant protein. Some genetic factors including the genetic variations in SPINK-1 protein have been linked to chronic pancreatitis and diabetes. We therefore hypothesized that SPINK-1 mutations might be a risk factor for the development of NAFLD.
Methods: Liver biopsy proven fifty NAFLD cases (20 steatohepatitis, 30 diffuse fatty liver disease and 44 healthy controls were included to the study. Liver function tests were measured. Body mass index was calculated. Insulin resistance was determined by using a homeostasis model assessment (HOMA-IR). Ultrasound evaluation was performed for each subject. Common genetic mutations in the third exon of SPINK-1 gene were analyzed by direct sequencing method.
Results: We found two cases with a SNP at N34S location in NAFLD group (allele frequency %4). One subject with diffuse fatty liver disease and other with liver cirrhosis due to NAFLD had N34S mutation. No SNPs were detected in healthy controls. In conclusions, in limited number of patients SPINK-1 mutations were not considered as a risk factor alone for NAFLD development.
Non-alcoholic fatty liver disease (NAFLD) Serine protease inhibitor Kazal-1 (SPINK-1) Homeostasis model assessment (HOMA-IR) Body Mass Index (BMI)
IntroductionNon-alcoholic fatty liver disease (NAFLD) is common in obese and its association with insulin resistance and diabetes has been reported.1,2 Whereas most individuals with nonalcoholic fatty liver disease will have steatosis, only a minority will develop steatohepatitis or cirrhosis.3 Family studies and interethnic variations in susceptibility to NAFLD suggest that genetic factors may be important in determining disease risk and progression.
Serine Protease Inhibitor Kazal-1 (SPINK-1) is an effective antiprotease protein mostly active against pancreatic trypsinogene.4 There are common described mutations in the third exon of SPINK-1 gene including N34S and P55S.5 SPINK-1 mutations were found to be a risk factor for chronic pancreatitis development.6,7 Additionally, common SPINK-1 mutation located at third exon named N34S was reported to be associated with diabetes. This effect was independent from the presence of chronic pancreatitis.8 Non obese persons with SPINK-1 mutation have an early onset type 2 diabetes suggesting that SPINK-1 mutations increases insulin resistance even in subjects with low body mass index (BMI).9
Experimental studies proved that SPINK-1 protein was highly synthesized in liver and adipose tissue of obese or diabetic rats.10 SPINK-1 protein also acts as an acute phase protein released in different inflammatory conditions.10 Those findings suggest that SPINK-1 gene mutations might play a pivotal role in development of insulin resistance and in turn liver steatosis or steatohepatitis. This study aimed to investigate the role of common SPINK-1 mutations in NAFLD development.
Material and methodsConsecutive patients admitted to Ege University Hepatology Outpatient Clinic between 2002-2006 with elevated liver function tests, no alcohol history, negative viral or autoimmune serology were evaluated further for NAFLD. Patients with positive viral serology, autoimmune diseases, cholestatic liver disorders, metabolic liver diseases or suspected toxic hepatitis, chronic pancreatitis, gastrointestinal operation, any systemic disorders, type 1 diabetes and hyperlipidemia were excluded. Informed consent in writing was obtained from each patient and the study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Ultrasound evaluation was performed for each subject. Body mass indexes were calculated. Insulin resistance was determined by using a homeostasis model assessment (HOMA-IR). Liver biopsy was performed in patients with elevated liver function tests. Biopsy proven 50 NAFLD patients was included to the study. Healthy volunteers (n 44) were evaluated as a control group.
Common genetic mutations in the third exon of SPINK -1 gene was analyzed by direct sequencing method. Genomic DNA was extracted from peripheral blood leukocytes by standard protocols (Qiagen DNA extraction kit).11 Based on the published nucleotide sequence of SPINK 1 gene, primers flanking the third exon of SPINK 1 gene were used for PCR as described in the literature (11). PCR conditions was as follow: Ampli Taq Gold polymerase (0.5 U; Perkin Elmer), deoxynucleoside triphosphates (400 • mol/L) and primer (0.1 • mol/L each) in a total volume of 25 • L. Cycle conditions were as follows: initial denaturation for 12 min at 95 °C; 36 cycles of 15 s denaturation at 95 °C, 30 s of annealing at 56 °C and 30 s of primer extension at 72 °C; and a final extension for 2 min at 72 °C. Purified PCR products with spin columns were evaluated by direct sequencing (ABI 3300, Big-Dye terminator sequencing protocol).
DNA sequences were analyzed by sequencer analysis program. Prevalence of genetic mutations was given as allele frequency. Demographic and biochemical results were given as mean and SD. Armitage trend test and student’s t test were used for statistical analysis. P < 0.05 was considered as statistically significant.
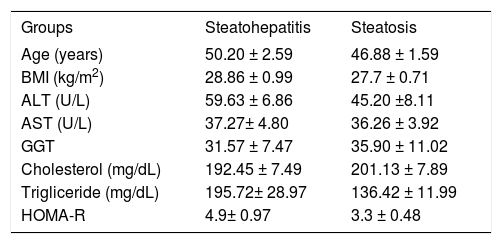
ResultsFifty NAFLD subjects were included to the study. The age and gender distribution was similar between NAFLD group and healthy controls. Liver biopsies proved the diagnosis of steatohepatitis in 20 and diffuse steatosis in 30 subjects. Patient’s characteristics were given in Table I. Liver functions tests of NAFLD group was significantly higher than controls. Patients with steatohepatitis or steatosis had high HOMA-R values demonstrating the presence of insulin resistance.
General characteristic of NAFLD patients.
| Groups | Steatohepatitis | Steatosis |
|---|---|---|
| Age (years) | 50.20 ± 2.59 | 46.88 ± 1.59 |
| BMI (kg/m2) | 28.86 ± 0.99 | 27.7 ± 0.71 |
| ALT (U/L) | 59.63 ± 6.86 | 45.20 ±8.11 |
| AST (U/L) | 37.27± 4.80 | 36.26 ± 3.92 |
| GGT | 31.57 ± 7.47 | 35.90 ± 11.02 |
| Cholesterol (mg/dL) | 192.45 ± 7.49 | 201.13 ± 7.89 |
| Trigliceride (mg/dL) | 195.72± 28.97 | 136.42 ± 11.99 |
| HOMA-R | 4.9± 0.97 | 3.3 ± 0.48 |
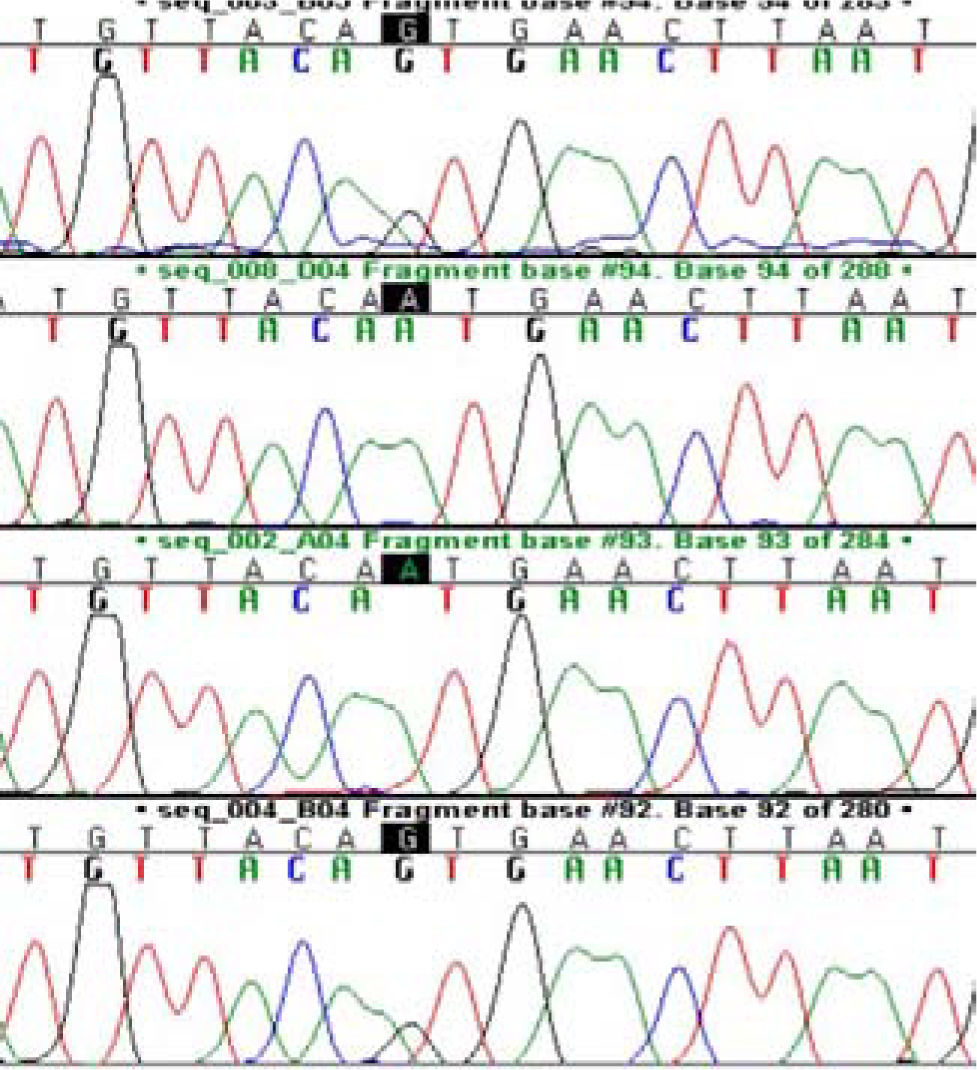
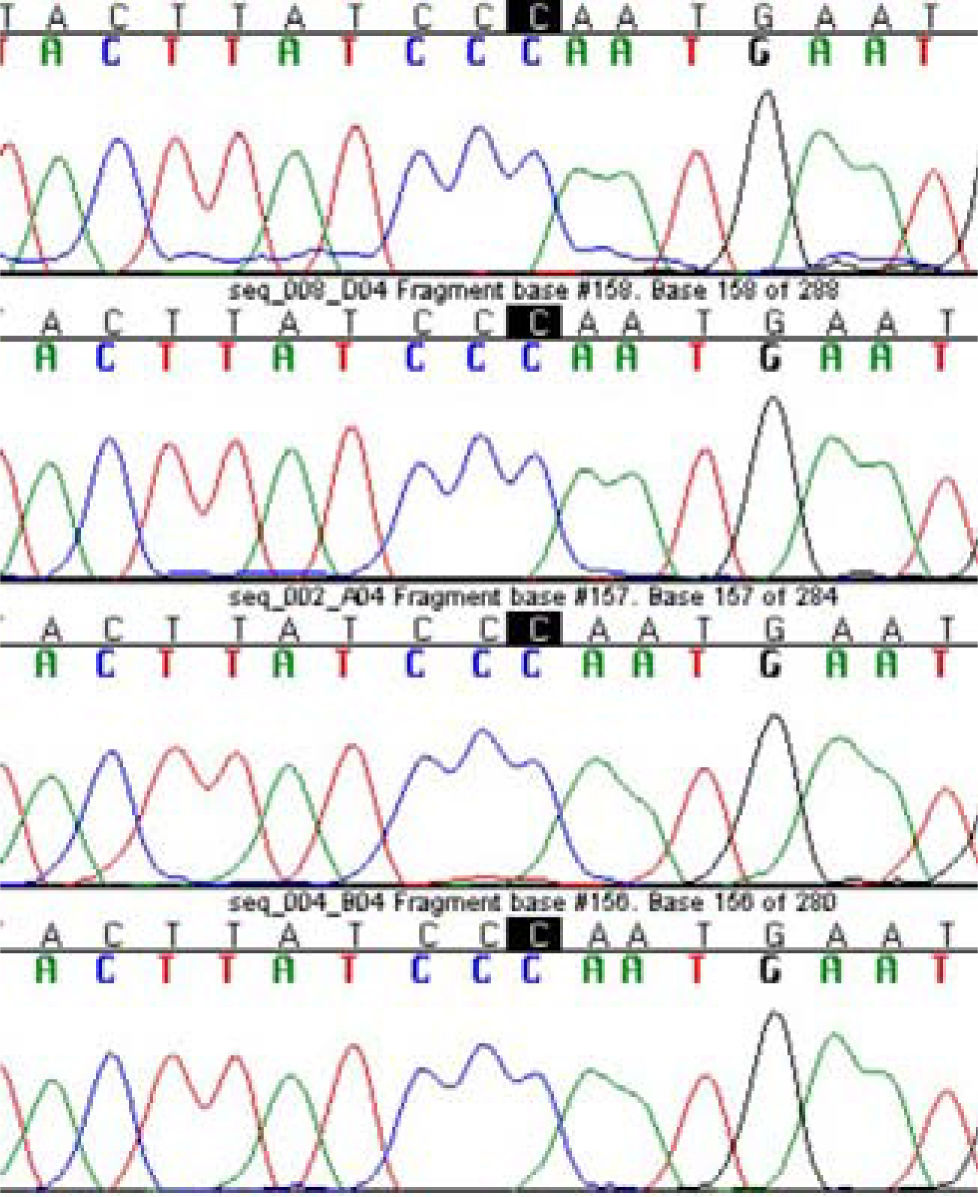
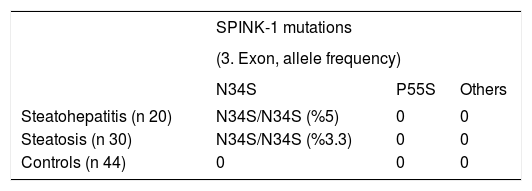
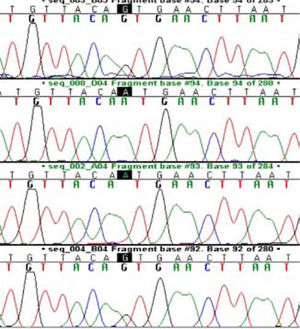
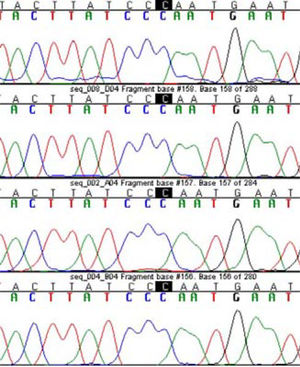
Two patients; one with diffuse steatosis, and one with liver cirrhosis secondary to steatohepatitis had homozygote mutation at N34S location (Figure 1). Those two patients had HOMA-R values higher than 3 implying the presence of insulin resistance. Calculated allele frequency was 4% in NAFLD. Neither P55S mutation nor any other new mutations were found in SPINK-1 gene third exon of NAFLD patients (Figure 2). The total allele frequency of mutations in SPINK-1 gene, 3 th exon were 5% in steatohepatitis and 3.3% in steatosis groups. The difference was not significant. None of the controls had any mutation at third exon of SPINK -1 gene (Table II).
DiscussionNonalcoholic fatty liver disease (NAFLD) has wide spectrum of symptoms extending from simple steatosis, through nonalcoholic steatohepatitis (NASH) to cirrhosis and hepatocellular carcinoma.12 Family studies suggest that genetic factors might be an important factor in NAFLD development.13,14 Several approaches can be used to look for genetic factors playing a role in NAFLD. Factors influencing the risk factors for NAFLD are candidates for further genetic research.
The most accepted risk factors for NAFLD are insulin resistance, obesity and diabetes.15,16 The genetics underlying Type 2 diabetes mellitus is complex, and it is likely that several genes act in concert with non-genetic factors. Recently, Schneider et al reported an association of mutations in the SPINK-l gene with early-onset Type 2 diabetes mellitus in patients from Bangladesh.8 They found the SPINK-1, N34S mutation in 14% patients with diabetes. Similar to that, Hassan et al. detected the N34S mutation in patients from southern Asia with early onset diabetes; implying its effect on development of insulin resistance.9
Though its associations with insulin resistance and diabetes, the role of SPINK-1 mutation in NAFLD has not been investigated previously. This study showed that %4 of NAFLD subjects had mutation at N34S in SPINK-1 gene. No other SPINK-1 genetic mutations were found in NAFLD patients or healthy controls. SPINK-1 N34S mutation is common worldwide, with an incidence ranging from 1 to 4% of the general population.11Since we selected only the liver biopsy proven NAFLD patients we evaluated the limited number of patients leading to possibility of type 2 error. However since the allele frequency in NAFLD were similar to previously reported frequencies in European countries11we suggest that the sample was representative of the larger population. We found that SPINK1 mutation is not a risk factor alone for NAFLD development in Turkish population. None of the healthy subjects in our study had SPINK-1 mutation suggesting that those mutations are rare in Turkish population.
Serine protease inhibitor protein was purified from rat hepatocytes previously.17 Similarly liver tissues from non-hepatitis, chronic hepatitis and cirrhosis patients revealed that up to 40% express pancreatic secretory trypsin inhibitor. Omachi et al found that serine protease inhibitor gene was expressed in all hepatocellular carcinomas, but not in other tumors, including mammary, thyroid, pulmonary and ovarian cancers.18,19 These observations suggest that the progression of NAFLD to hepatocellular carcinoma might be driven by SPINK-1 gene. However testing this hypothesis in our limited study group was not possible since none had hepatocellular carcinoma. SPINK1 gene expression in NAFLD, hepatocellular carcinoma and other chronic liver disorders should be evaluated further.
In conclusion, we found that SPINK-1 mutations were rare in NAFLD and were not considered as a risk factor alone for disease development in Turkish population. Further studies in different populations might enlighten the role of SPINK-1 gene mutations and expression in NAFLD.