Alcoholic hepatitis (AH) is a condition of acute liver inflammation in the setting of heavy alcohol use that is often managed with corticosteroids in severe cases. Among non-responders to steroids, however, prognosis is poor with up to 75% mortality within 6 months after treatment failure. Early liver transplantation (LT) can achieve an acceptable short-term survival, and initial studies have demonstrated 3-year survival rates of up to 84%. However, the practice of early LT in severe AH remains controversial with concerns over the 6-month rule of sobriety and risk of alcohol relapse post-transplant. Proponents of LT advocate for better understanding of alcohol use as a disorder rather than self-inflicted cause of illness, aim to redefine the misguided application of the 6-month rule, and point out similar relapse rates among patients with early LT and those with greater than 6 months abstinence before transplant. Opponents of LT emphasize the correlation between alcohol relapse and graft failure and mortality, public resistance and potential for distrust among donors, and arguments that transplant centers need to establish improved models to predict relapse and standardize candidate selection criteria across centers. Here we review recent literature on this controversy and provide recommendations for moving forward to consensus.
Alcoholic hepatitis (AH) is a syndrome characterized by acute inflammatory liver injury in the setting of excessive alcohol use. Diagnosis of AH may be determined clinically in the setting of acute jaundice, fever, malaise, weight loss and malnutrition, elevation of aspartate ami-notransferase (AST) greater 1.5–2.0 times that of alanine aminotransferase (ALT), with AST ranges usually > 50 to 300 IU/mL.1 Recent guidelines have recommended a standard definition of jaundice within the last 8 weeks and ongoing heavy alcohol use for at least 6 months, though some patients may demonstrate less than 60 days abstinence before onset ofjaundice.2 Heavy alcohol use leading to alcohol-related liver disease has been described as consumption of > 20 g per day in women and > 30 g per day in men.1 The mechanism of liver injury is attributed to the production of pro-inflammatory cytokines by Kupffer cells, induced by lipo-polysaccharide translocation secondary to increased gut permeability.3,4 Histologically, this results in steatosis, hepatocyte ballooning, and neu-trophilic infiltration. Liver biopsy may be used as a diagnostic adjunct in patients with atypical presentation, uncertain alcohol history, or presence of other confounding factors.5
In cases of severe AH, treatment with steroids confers a modest survival benefit.5,6 However, studies have demonstrated that as many as 40% of patients treated with steroids may be non-responders. These patients subsequently have a 6-month mortality rate as high as 75%, without the option for second-line medical therapy.7,8 For these patients, liver transplantation (LT) provides a reasonable rescue therapy. However, transplant centers have traditionally required 6 months abstinence before liver transplantation, known as the “6-month rule.” By definition, patients with severe AH would not meet this time requirement before diagnosis, steroid failure, and need for rescue therapy. As such, early experiences in liver transplantation before 6 months sobriety in patients with alcoholic hepatitis have demonstrated improved post-transplant survival of 77-100% at 6 months, among those who receive LT compared to those treated with supportive care.9–13 Given the success of early studies, controversies exist regarding this strategy due to concerns of limited availability of donor organs, risk of relapse after LT, and lack of standardized policy across transplant centers.
The aim of this review article is to summarize commonly utilized prognostic models in severe AH, the role of steroids as pharmacologic treatment, and early LT for severe AH patients non-responsive to steroids. We aim to discuss survival rates with and without transplantation, relapse rates after LT, and arguments in favor and against utilizing this therapeutic intervention.
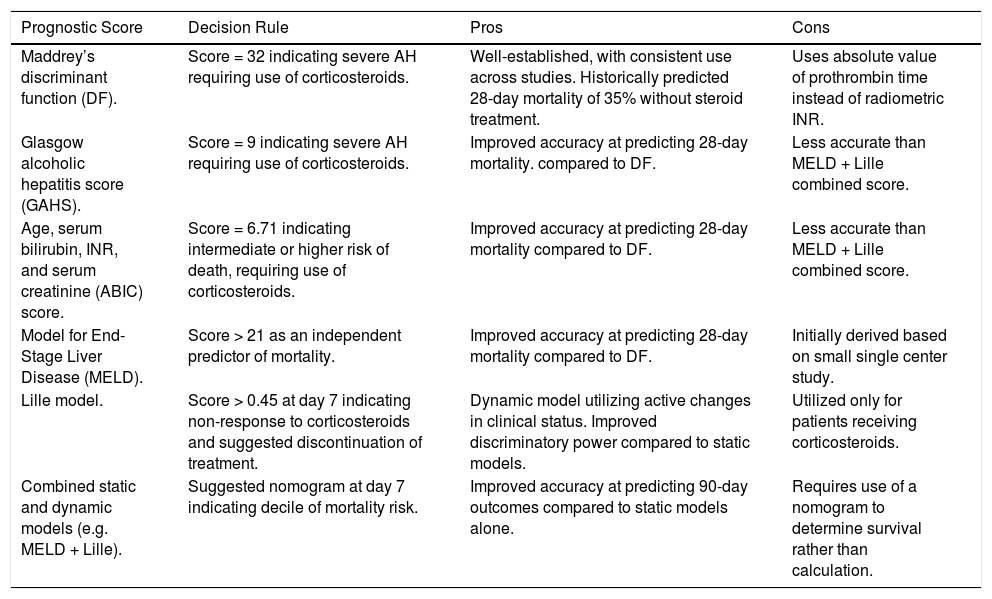
Prognostic ModelsSeveral scoring systems have been developed to evaluate the severity of AH, determine prognosis, and guide treatment decisions. The most established and commonly utilized prognostic model is Maddrey’s discriminant function (DF), with a score ≥ 32 indicating severe AH requiring treatment with corticosteroids.14 Historically, combined analysis has shown a predicted 28-day mortality rate of 35% in patients who do not receive treatment with steroids,15 though more recent studies suggest the mortality rate may be closer to 15–20%.16 Critics have expressed concern regarding the utility of the DF score, as it is calculated using the absolute value of prothrombin time rather than international normalized ratio (INR). As a result, other scoring systems have been proposed as alternatives.
The Glasgow alcoholic hepatitis score (GAHS) utilizes age, serum bilirubin and blood urea on day 1, and serum bilirubin, prothrombin time, and peripheral white blood cell count on day 6–9, to predict 28-day outcomes. Severe AH is classified by GAHS > 8, and early studies demonstrated an accuracy of 81% compared to a lower performing prediction of 49% by the DF score.17 Another system, known as the age, serum bilirubin, INR, and serum creatinine (ABIC) score, emerged to stratify patients according to risk of mortality at 90 days and 1 year. Low, intermediate, and high risk of death was characterized by scores of < 6.71, 6.71 – 8.99, and > 8.99, respectively.18
The Model for End-Stage Liver Disease (MELD) score can also be utilized to predict mortality in severe AH. Dunn, et al., performed a retrospective cohort study to determine the 30-day and 90-day mortality for 73 patients hospitalized with AH at a single center.19 The authors determined that a MELD score of 21 or greater was an independent predictor of 90-day mortality, and furthermore that both DF and MELD scoring systems were similar in predicting mortality at 30 and 90 days.19
In contrast to these “static” scoring systems, based on one time point during treatment, other “dynamic” scoring systems have been designed to include the change in bilirubin and assess benefit from therapy. For instance, the Lille model incorporates age, creatinine, albumin, prothrombin time, bilirubin level at day 0, and the serum bilirubin level at day 7.7 Patients with a score above 0.45 are deemed to be “non-responders” to corticosteroid therapy, indicating that clinicians should consider discontinuing therapy given lack of improvement in liver function and greater risk of mortality from infection-related complications.7 In a separate study, Louvet, et al., combined the static models (Maddrey DF and MELD) with the dynamic Lille model to predict survival at 2 and 6 months after presentation, and the MELD + Lille combination was found to be the best predictive model of expected mortality.20 Mortality at 6 months can be determined by utilizing a nomogram at day 7 of treatment, which provides deciles regarding mortality risk when combining these scoring systems.20
A recent study examining the performance of existing scoring systems demonstrated that GAHS, ABIC, and MELD are superior to DF in predicting 28-day survival benefit from prednisolone, with consistently low static scores indicating favorable outcomes regardless of prednisolone use.21 However, it was noted that combining static with dynamic scoring systems such as the Lille model, had improved performance at predicting 90-day outcomes.21 A comparison of scoring systems is shown in table 1.
| Prognostic Score | Decision Rule | Pros | Cons |
|---|---|---|---|
| Maddrey’s discriminant function (DF). | Score = 32 indicating severe AH requiring use of corticosteroids. | Well-established, with consistent use across studies. Historically predicted 28-day mortality of 35% without steroid treatment. | Uses absolute value of prothrombin time instead of radiometric INR. |
| Glasgow alcoholic hepatitis score (GAHS). | Score = 9 indicating severe AH requiring use of corticosteroids. | Improved accuracy at predicting 28-day mortality. compared to DF. | Less accurate than MELD + Lille combined score. |
| Age, serum bilirubin, INR, and serum creatinine (ABIC) score. | Score = 6.71 indicating intermediate or higher risk of death, requiring use of corticosteroids. | Improved accuracy at predicting 28-day mortality compared to DF. | Less accurate than MELD + Lille combined score. |
| Model for End-Stage Liver Disease (MELD). | Score > 21 as an independent predictor of mortality. | Improved accuracy at predicting 28-day mortality compared to DF. | Initially derived based on small single center study. |
| Lille model. | Score > 0.45 at day 7 indicating non-response to corticosteroids and suggested discontinuation of treatment. | Dynamic model utilizing active changes in clinical status. Improved discriminatory power compared to static models. | Utilized only for patients receiving corticosteroids. |
| Combined static and dynamic models (e.g. MELD + Lille). | Suggested nomogram at day 7 indicating decile of mortality risk. | Improved accuracy at predicting 90-day outcomes compared to static models alone. | Requires use of a nomogram to determine survival rather than calculation. |
For severe AH, determined by Maddrey DF score ≥ 32, MELD score ≥ 21, or the presence of encephalopathy, the standard of care is treatment with prednisolone 40 mg daily for 28 days.1,19,22 The benefit of steroid treatment has been demonstrated by a meta-analysis of five randomized-control trials with a total of 418 patients, showing that those patients receiving prednisolone had higher 28-day survival rates compared to placebo (80 vs. 66%, p = 0.0005).15 The survival benefit was mainly observed in patients identified as responders per the Lille model.15 For patients with baseline renal insufficiency, pentoxyfilline has been studied as an alternative treatment,6 based on data from a randomized controlled trial demonstrating decreased inpatient mortality with pentoxyfilline compared to placebo (24.5 vs. 46.1%). The decreased mortality rate was deemed secondary to a reduction in development of hepatorenal syndrome.23
Despite concerns regarding the safety of steroids and efficacy of pentoxyfilline, the survival benefit of prednisolone was confirmed in the STOPAH trial.16 In this trial of 1,103 patients, multivariable analysis revealed that taking prednisolone improved 28-day survival (OR for death = 0.61, 95% CI 0.41-0.91). No benefit was seen with pentoxyfilline. Additionally, the effect of prednisolone on mortality at 90 days (OR = 1.00; 95% CI, 0.73 to 1.36; P = 0.98) and at 1 year (OR = 1.01; 95% CI, 0.74 to 1.39; P = 0.94) was not significant when compared to not taking prednisolone.16
Beyond traditional pharmacological treatment, other therapies have been studied, including enteral nutrition, immunotherapy, and extracorporeal liver therapy (ELAD). Moreno, et al., assessed intensive enteral nutrition therapy, defined by a regimen of enteral feeding tube for 14 days. Although methylprednisolone combined with intensive nutrition did not increase survival compared to conventional nutrition, authors identified that low daily caloric intake of less than 21.5 kcal/kg/day was associated with higher mortality, suggesting an overall significance of adequate nutrition.24 Immunotherapy such as granulocyte-colony stimulating factor (G-CSF) has been postulated to mobilize CD34+ stem cells, induce hepatocyte regeneration, and therefore improve survival. Spahr, et al., initially demonstrated in a small cohort of patients with alcoholic cirrhosis, an increase in hepatocyte growth factor and thus hepatic progenitor cells when combining a 5-day course of G-CSF to prednisone, compared to prednisone standard therapy alone.25 Additional studies have reiterated improved survival rates at 90 days (78.3 vs. 30.4%, P = 0.001)26 with associated reduction in MELD scores at 3 months by 55% (P = 0.01) with use of G-CSF, however these studies used pentoxifylline instead of prednisone as standard therapy for comparison.27 In a multinational, prospective, randomized controlled trial of 203 patients, investigators have tested extracorporeal liver therapy (ELAD) in severe AH, finding no difference in overall 90-day survival. However, subgroup analysis suggests possible benefits in a specific population with MELD < 28, age < 47, sufficient renal function, and less severe coagulopathy.28 These alternate interventions may serve a role in supplementing existing and current therapies, however they require larger clinical trials to validate overall efficacy.
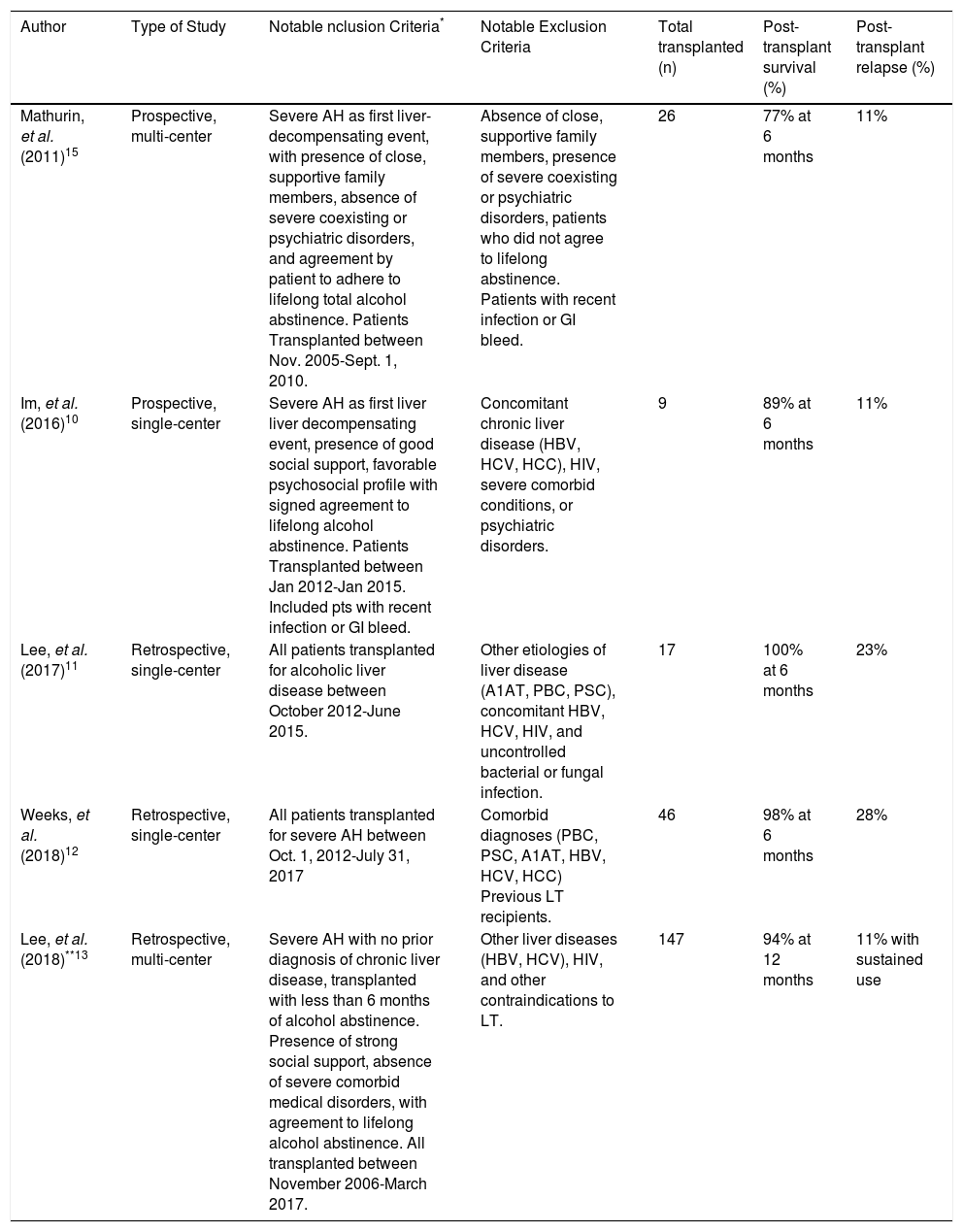
Outcomes After Early Liver TransplantationIn the subgroup of patients that fail to respond to corticosteroid therapy, the 6-month survival rate is approximately 25%, leading clinicians to consider early liver transplantation. Early studies of this practice are summarized in table 2.9–13 In 2011, Mathurin, et al. conducted the landmark European trial of early LT for patients with severe AH nonresponsive to medical.9 The authors identified a total of 26 patients through a stringent selection process consisting of several meetings among multiple medical teams, the patient’s family, and the patient. Selected patients were non-responders to medical therapy who presented with AH as the first liver-decompensating event and met strict psychosocial criteria, including presence of supportive family members, absence of coexisting or psychiatric disorders, and agreement by patients and family members to adhere to lifelong total abstinence. For selected candidates matched to controls, the 6-month survival rate was higher among early transplant recipients (77 vs. 23%, P < 0.001), and this benefit remained through 2 years of follow-up time (HR = 6.08, P = 0.004). Only 3 of the 20 recipients (12%) relapsed at 720, 740, and 1140 days post-transplant, with one returning to harmful levels of drinking (> 50 g/day).9
Summary of previous publications on early liver transplantation for alcoholic hepatitis.
| Author | Type of Study | NotabIe nclusion Criteria* | Notable Exclusion Criteria | Total transplanted (n) | Post-transplant survival (%) | Post-transplant relapse (%) |
|---|---|---|---|---|---|---|
| Mathurin, et al. (2011)15 | Prospective, multi-center | Severe AH as first liver-decompensating event, with presence of close, supportive family members, absence of severe coexisting or psychiatric disorders, and agreement by patient to adhere to lifelong total alcohol abstinence. Patients Transplanted between Nov. 2005-Sept. 1, 2010. | Absence of close, supportive family members, presence of severe coexisting or psychiatric disorders, patients who did not agree to lifelong abstinence. Patients with recent infection or GI bleed. | 26 | 77% at 6 months | 11% |
| Im, et al. (2016)10 | Prospective, single-center | Severe AH as first liver liver decompensating event, presence of good social support, favorable psychosocial profile with signed agreement to lifelong alcohol abstinence. Patients Transplanted between Jan 2012-Jan 2015. Included pts with recent infection or GI bleed. | Concomitant chronic liver disease (HBV, HCV, HCC), HIV, severe comorbid conditions, or psychiatric disorders. | 9 | 89% at 6 months | 11% |
| Lee, et al. (2017)11 | Retrospective, single-center | All patients transplanted for alcoholic liver disease between October 2012-June 2015. | Other etiologies of liver disease (A1AT, PBC, PSC), concomitant HBV, HCV, HIV, and uncontrolled bacterial or fungal infection. | 17 | 100% at 6 months | 23% |
| Weeks, et al. (2018)12 | Retrospective, single-center | All patients transplanted for severe AH between Oct. 1, 2012-July 31, 2017 | Comorbid diagnoses (PBC, PSC, A1AT, HBV, HCV, HCC) Previous LT recipients. | 46 | 98% at 6 months | 28% |
| Lee, et al. (2018)**13 | Retrospective, multi-center | Severe AH with no prior diagnosis of chronic liver disease, transplanted with less than 6 months of alcohol abstinence. Presence of strong social support, absence of severe comorbid medical disorders, with agreement to lifelong alcohol abstinence. All transplanted between November 2006-March 2017. | Other liver diseases (HBV, HCV), HIV, and other contraindications to LT. | 147 | 94% at 12 months | 11% with sustained use |
All patients met criteria for severe alcoholic hepatitis based on Maddrey’s discriminant function score = 32, and were deemed nonresponsive or ineligible for medical therapy.
This study encompasses data from the American Consortium of Early Liver Transplantation, which comprises 12 centers from 8 United Network for Organ Sharing regions. This comprehensive publication includes the results of single-center studies presented by Im, et al.,10Lee, et al.11and Weeks, et al.12
In the United States, Im, et al., reported a single-center experience of early liver transplantation.10 The authors identified a total of 9 non-responders to medical therapy, who also presented with severe AH as the first liver-de-compensating event, had good social support, and were deemed to have a favorable psychosocial profile with low relapse risk. Candidate selection followed the process typical of selection committee meetings, comprising staff hepatologists, transplant psychiatrists and social workers, senior hepatologists, transplant surgeons, and financial coordinators, with consensus required before listing for early LT. For selected patients matched to controls, 6-month survival rates were higher among early transplant recipients (89 vs. 11%, p < 0.001). After transplantation, only one patient relapsed. Although the presence of good social support was a general requirement for a favorable psychosocial profile, the authors found it had minimal impact on candidate selection. Multivariable analysis instead demonstrated that good insight into the consequences of alcohol use, along with severe AH as the first liver-decompensating event was significantly associated with selection for transplant listing.10
Lee, et al., published another single-center trial of LT in the United States for patients with alcohol-related liver disease, stratifying patients into two groups: 1) severe AH as the first liver decompensation event, and 2) alcoholic cirrhosis (AC) with ≥ 6 months abstinence.
A total of 17 patients with AH and 26 patients with AC underwent liver transplantation. Six-month survival rates were 100% for AH and 89% for AC (P = 0.27), with alcohol relapse rates numerically but not statistically higher among AH patients (23.5 vs. 11.5%, p = 0.42).11 Long-term follow-up on a larger cohort of patients from this initial study was presented in a different publication by Weeks, et al., comparing 46 patients with AH and 34 patients with AC. After a median follow-up of 532 days post-transplant, there were no significant differences between groups in 6-month and 1-year patient or graft survival. Alcohol relapse was also similar, found to be 28% in the AH group compared to 24% in the AC group.12
More recently, Lee, et al., published findings from the multicenter, American Consortium of Early Liver Transplantation, which comprises 12 centers from 8 United Network for Organ Sharing regions.13 This comprehensive publication includes the results of single-center studies presented by Im, et al.,10 Lee, et al.11 and Weeks, et al.12 Across all centers, there were 147 transplants performed for patients who presented with severe AH as the first decompensating event. Among these patients, the median number of days for listing before LT was 7 (range 3 – 12), and the median number of days from the last alcoholic drink to LT was 55 (range 36 - 91). One-year survival was 94% (95% CI 89 – 97%), and three-year survival was 84% (95% CI 75 – 90%). Cumulative incidence of any alcohol use was 25% at 1-year post-transplant and 34% at 3 years, however cumulative incidence of sustained alcohol use was lower, at 10% at 1 year and 17% at 3 years post-transplant. Sustained alcohol use post-transplant was associated with increased risk of mortality at HR 4.59.13
Predictors of post-LT relapse were younger age (OR 1.05, CI 1.01-1.09, p = 0.02) and lack of complete acceptance of diagnosis (OR 2.36, CI 1.10-5.08, p = 0.04). Notably, factors not associated with post-transplant alcohol use included length of pre-LT abstinence, quantity of alcohol use, years of heavy drinking, history of illicit drug use, or history of failed rehabilitation attempts. The greatest predictors of post-LT death included sustained alcohol use post-LT (HR 4.59, CI 1.45-14.54, p = 0.01), any alcohol use post-LT (HR 3.54, CI 1.06-11.85, p = 0.04), and > 10 drinks per day at presentation (HR 3.17, CI 1.04-9.67, p = 0.04). Length of pre-LT abstinence and other alcohol-related factors, as well as pre-LT clinical factors such as Maddrey DF score, Lille score, and MELD scores were not associated with post-LT death.13
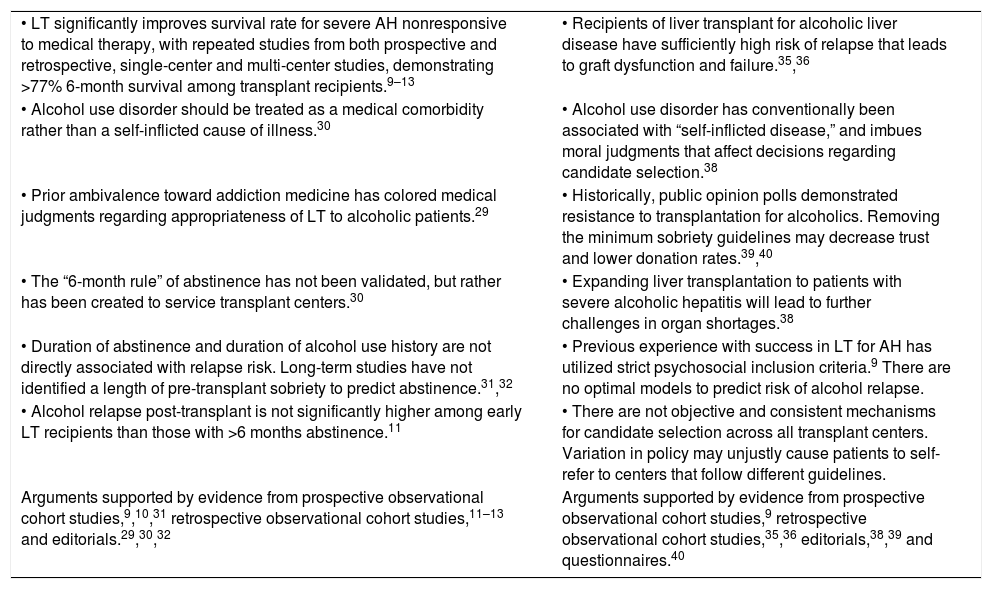
The ControversyWith studies demonstrating positive outcomes, early transplantation for AH patients is an important rescue therapy. However, attitudes and practices regarding liver transplantation are divided along those in favor of early transplant and those who believe risks outweigh the benefits of transplant (Table 3).
Opinions regarding early liver transplantation for alcoholic hepatitis.
| • LT significantly improves survival rate for severe AH nonresponsive to medical therapy, with repeated studies from both prospective and retrospective, single-center and multi-center studies, demonstrating >77% 6-month survival among transplant recipients.9–13 | • Recipients of liver transplant for alcoholic liver disease have sufficiently high risk of relapse that leads to graft dysfunction and failure.35,36 |
| • Alcohol use disorder should be treated as a medical comorbidity rather than a self-inflicted cause of illness.30 | • Alcohol use disorder has conventionally been associated with “self-inflicted disease,” and imbues moral judgments that affect decisions regarding candidate selection.38 |
| • Prior ambivalence toward addiction medicine has colored medical judgments regarding appropriateness of LT to alcoholic patients.29 | • Historically, public opinion polls demonstrated resistance to transplantation for alcoholics. Removing the minimum sobriety guidelines may decrease trust and lower donation rates.39,40 |
| • The “6-month rule” of abstinence has not been validated, but rather has been created to service transplant centers.30 | • Expanding liver transplantation to patients with severe alcoholic hepatitis will lead to further challenges in organ shortages.38 |
| • Duration of abstinence and duration of alcohol use history are not directly associated with relapse risk. Long-term studies have not identified a length of pre-transplant sobriety to predict abstinence.31,32 | • Previous experience with success in LT for AH has utilized strict psychosocial inclusion criteria.9 There are no optimal models to predict risk of alcohol relapse. |
| • Alcohol relapse post-transplant is not significantly higher among early LT recipients than those with >6 months abstinence.11 | • There are not objective and consistent mechanisms for candidate selection across all transplant centers. Variation in policy may unjustly cause patients to self-refer to centers that follow different guidelines. |
| Arguments supported by evidence from prospective observational cohort studies,9,10,31 retrospective observational cohort studies,11–13 and editorials.29,30,32 | Arguments supported by evidence from prospective observational cohort studies,9 retrospective observational cohort studies,35,36 editorials,38,39 and questionnaires.40 |
Opinions in favor of LT primarily focus on the separation between alcohol use as a disorder and alcohol use as a cause of liver disease. Lucey describes this differentiation that alcohol use disorder is an illness that may itself be complicated by alcoholic liver disease.29 Alcohol use disorder and addiction are not purely subjective choices, but rather involve multifactorial mechanisms including genetics, neurohormonal communication, the microbiome, and environmental factors.30 Alcohol use should therefore be viewed similarly to other chronic medical comorbidities such as diabetes mellitus or hypertension, and weighed as a medical diagnosis that can impact suitability for LT. Until now, ambivalence about addiction and treatment of addiction as a self-inflicted disorder that patients can “control on their own,” has influenced medical judgments regarding the appropriateness of alcoholic patients for LT. This has also been reflected in terminology, using the word “recidivism,” which has origins is legal phrasing that implies personal fault, instead of “recurrence”.29 To fully understand a candidate’s addiction to alcohol as a disease process as opposed to “lack of willpower” requires engaging with addiction specialists.
Beyond the definition of alcohol use disorder, the 6-month rule for sobriety has been brought under question. It is argued that current application of requiring 6 months sobriety before transplantation has been used out of context from its original intent, which was to identify patients who could obtain spontaneous recovery with improvement of liver function after a period of abstinence.30 Previously, the 6-month period was proposed as a surrogate predictor of alcohol relapse. However, it has been found that duration of abstinence is not a consistent predictor.31 Although longer duration of sobriety has been associated with a reduction in risk of “any alcohol use” after transplant, studies have not distinguished between return to harmful levels of drinking compared to minimal levels. In long-term studies of alcohol abstinence, risk of relapse is less likely after 5 years of sobriety.32 Time period alone does not account for other predictors such as co-existing psychiatric illness, other substance abuse, and previous efforts at sobriety or rehabilitation.30 As such, the “6-month rule” has not been validated, but rather has been created to service transplant centers, selection committees, and insurance companies by providing simple rules to define candidacy for listing. Predicting relapse after transplantation is complex and challenging, and applying a blanket rule for all patients would unfairly exclude many with potential to benefit from early LT.
In terms of mortality outcomes, not only does LT significantly improve survival for severe AH refractory to medical treatment, but post-transplant survival rates and relapse rates are similar between those transplanted for AH as compared to chronic alcoholic liver disease.33,34 Lee, et al., demonstrated this comparison by identifying similar alcohol relapse rates between patients transplanted with AH and patients transplanted with alcoholic cirrhosis and 6 months abstinence.11 Enforcement of the “6-month rule” may therefore delay listing among those who would otherwise die without LT, even if unlikely to relapse after LT.9
Together, the evidence provided by early experience with liver transplantation for severe AH along with arguments for better understanding alcohol use disorder and the need for validating the 6-month rule, suggest that early liver transplantation should be more widely accepted as a rescue therapy among select patients.
Against Early TransplantationAlthough early transplantation is clearly beneficial to many patients with alcoholic hepatitis, opponents of this practice note the high rate of recurrent harmful drinking and resulting graft dysfunction. In a study of 369 patients transplanted with alcoholic liver disease, 20% showed severe relapse with approximately one third of this subset getting a recurrence of alcoholic cirrhosis at a median time of 6 years (range 3 to 10) from LT to cirrhosis.35 Another study showed that long-term survival was significantly reduced in those that relapsed compared to those that remained abstinent (45 vs. 86% at 10 years, P < 0.01).36 Both of these studies demonstrate study populations at-risk for relapse and resulting graft failure and dysfunction. This should be considered in the context of the overall role of alcohol use and all-cause mortality, with previous studies suggesting that people with alcohol use disorder have a four-fold increased risk from premature death and average 24- to 28-year decrease in life expectancy than the general population.37 Mortality rate ratios have also been noted to be highest among younger age groups, especially between 30-59 years old.37 Thus, in addition to concern for graft failure, alcohol use can itself increase risk of death from other medical conditions, suicide, and external causes of death.
Given the limited supply of donor organs, some argue that transplantation should be reserved for patients without such “preventable” risk factors for poor outcome. Alcohol use has conventionally been associated with “self-inflicted disease.” It remains that alcohol use disorder is viewed by both medical and moral principles, and despite efforts to isolate patients in terms of their medical diagnoses, moral judgments still impact decision making during LT selection.38 In addition to ethical considerations, the natural history of alcoholic hepatitis is variable. Statistically, 25–30% of AH patients may recover their liver function even without response to steroids.7 Thus, it is possible that up to 30% of transplants performed for severe AH will be done in patients who would have spontaneously recovered without liver transplant.
Beyond concerns about justice in allocating a limited supply of organs, others fear that expansion of early transplantation for AH may actually decrease organ donation rates by eroding public trust.39 Historically, public opinion polls consistently demonstrated resistance to transplantation for alcoholics in general,40 though a more recent survey suggests the public may be moving toward more neutral viewpoints regarding the specific role of early LT in severe alcoholic hepatitis.41 Given different practices among transplant centers and inconsistency among which centers employ the 6-month rule and which do not, it is difficult to project what the real impact of early LT for AH will be on donor pool. However, the burden of this practice will still lead to further challenges in organ shortages. Though only 3% of liver grafts were used for patients with AH in the earliest study by Mathurin, et al.,9 removing the minimum sobriety requirement and expanding LT to include this group will thereby increase the percentage of donor grafts needed.38
Finally, and perhaps most importantly, allowing liver transplantation for AH would require an objective and consistent mechanism for candidate selection across centers. Although the denominators are not clearly reported in the studies quoted above, the candidates selected for transplantation seem to represent ≤ 10% of all patients with severe AH at these centers. In addition, anecdotal reports suggest that many patients with AH are never referred to transplant centers in the first place. Thus, transplant programs would need to be prepared to handle a large volume of referrals, many of them critically ill, and perform rapid psychosocial evaluations. Such an evaluation, performed by an experienced team of social workers, substance abuse experts, and psychiatrists can sometimes identify high-functioning patients with good social support and good insight who have a fairly low risk of relapse despite a short period of sobriety. The problem is that these evaluations are subjective and difficult to perform rapidly. An accurate assessment is particularly challenging in patients with encephalopathy. Employing the practice of early LT may leave gastroenterologists in the community without clear guidelines for referral among patients with alcoholic liver disease. From the referral process to evaluation and selection, transplantation for this population would be further challenged by ethical and moral dilemmas regarding patients that may subsequently self-refer to centers that follow different guidelines for early LT.
The 6-month rule of sobriety defined by a 1997 consensus conference42 is often criticized as arbitrary and only weakly predictive of the likelihood of relapse. Although these criticisms are valid, in its defense the rule is objective and transparent. According to Norman Daniels, one of the most influential modern experts on distributive justice, even if we never achieve consensus on the criteria for resource allocation, we must ensure that the process is perceived as being fair.43
Thus, critics point to graft failure and overall increased mortality with alcohol relapse, the need for appropriate allocation, and the current challenge of inconsistent mechanisms for selection, as barriers to current practice in distributing scarce organs in resource-limited settings.
ConclusionsCurrent clinical guidelines regarding the management of AH focus on abstinence and medical treatment with corticosteroids.6 There is sufficient evidence to support similar or improved survival rates for early liver transplantation for severe alcoholic hepatitis nonresponsive to steroid therapy. However, the most common concerns are supported by cases of alcohol relapse after transplant, leading to graft failure. Attitudes toward early LT remain divided, with proponents advocating for a better understanding of alcohol use as a disorder, redefining the misguided application of the 6-month rule, and highlighting comparable relapse rates among recipients with less than and greater than 6 months sobriety. Meanwhile, opponents cite post-transplant relapse leading to reduced survival rates, ambivalent public opinions and concerns for a limited donor pool, and inconsistency in practices across transplant centers.
Both sides can agree on the need for optimizing candidate selection. Moving forward, further studies are needed to better define tools for predicting alcohol relapse, as well as methods to implement practical addiction resources and programs during post-transplant surveillance. At this time, there are still significant differences in practices of liver transplantation for alcoholic hepatitis across transplant centers. It is necessary to develop a united policy that settles the controversies regarding early LT for this population and begins to move toward broader consensus.
Abbreviations- •
AC: alcoholic cirrhosis.
- •
AH: alcoholic hepatitis.
- •
AST: aspartate aminotransferase.
- •
ALT: alanine aminotransferase.
- •
LT: liver transplantation.
- •
DF: Maddrey’s discriminant function.
- •
MELD: Model for End-Stage Liver Disease.
- •
UNOS: United Network for Organ Sharing.
- •
US: United States.
The authors have nothing to disclose regarding conflicts of interest relevant to this manuscript.
Financial SupportNone.
Writing AssistanceNone.
Author ContributionsTW, TM, MV, and VS drafted the manuscript. TM, AK, MV, SS, and VS provided critical revision of the manuscript for intellectual content.