Introduction and aim. Salidroside and curcumin (SC) formula could alleviate lipid deposition in high fat diet-induced nonalcoholic fatty liver disease (NAFLD). However, the mechanisms are still unknown, and the magnitude of potential therapeutic benefit remains understudied.
Material and methods. The rats were treated with high fat diet for 14 weeks to induce NAFLD. The experiment was divided into control, model (NAFLD), SC formula and rosiglitazone groups (n = 7 in each group). Hematoxylin-eosin (H&E) staining was applied to detect liver morphological changes. Biochemical, metabolic indices and inflammation factors in liver tissue and serum were detected. Additionally, the activities of related enzymes were detected by enzyme-linked immunosorbent assay.
Results. In the established rat model, typical lipid deposition and liver steatosis were observed. Liver triglyceride, free fatty acids, sera alanine aminotransferase, aspartate aminotransferase, gamma-glutamyl transferase, fasting insulin, fasting blood glucose and homeostasis model assessment of insulin resistance were elevated in model group. Liver malondialdehyde was significantly elevated, while superoxide dismutase was significantly decreased in model group, compared with control. Moreover, tumor necrosis factor-α and Interleukin-1 were significantly prod uced in model group, compared with control. As a mechanism, high fat diet decreased tissue AMP-activated protein kinase (AMPK), phosphorylated AMPK, carnitine palmitoyltransferase 1 and increased inacetyl-CoA carboxylase (ACCase), phosphorylated ACCase. Importantly, these abnormal changes caused by high fat diet were reduced by SC formula administration.
Conclusion. SC formula could ameliorate the injury caused by high fat diet. The effect was likely mediated via its influence on insulin resistance, lipid peroxidation injury and AMPK signaling pathway.
Nonalcoholic fatty liver disease (NAFLD) is a nonalcoholic-related liver disease manifested by lipid deposition and liver pathological changes.1 With the changes of life pattern and conditions, the incidence of NAFLD is in a rising trend. The incidence of NAFLD is 25–30% in USA, 12.6–50% in Asia countries, and 3–10% in children globally.2–6 Therefore, unravelling the pathogenetic mechanisms of NAFLD and developing novel therapeutic strategies for NAFLD are key imperatives.
NAFLD has the common features as obesity, dyslipidemia and diabetes.7 Similar to other diseases, such as hypertension, atherosclerosis and coronary heart disease, NAFLD also belongs to metabolic syndromes.8 Multiple factors could contribute to the aggravation of the disease, including inflammation reaction, lipid peroxidation injury, insulin resistance, etc.9 The chemical compound with single therapeutic target is inefficient to treat the metabolic diseases. The drugs with multiple pharmacological potential and combination therapy are supposed as the potential candidate treatment for HAFLD.10
Traditional Chinese medicine (TCM) is an alternation for the treatment of diseases because its multiple-target and -signaling pathway efficacy.11 TCM- salidroside and curcumin (SC) formula is composed of salidroside and curcumin, which are the major active components of TCM rhodiola rosea and rhizoma curcumae longae, respectively. Previous study revealed that SC formula had excellent advantages in ameliorating lipid deposition in NALFD rats.12 Compared with the single component, SC formula displays synergistic and additional effects.12 However, the magnitude of potential therapeutic benefit, as well as the mechanisms involved, remain uncertain.
In this study, we established NALFD model and evaluated the preventive effects of SC formula on high fat diet-induced liver injury. The potential mechanisms, including insulin resistance, lipid oxidative stress and the changes of AMPK signaling pathways were screened.
Material and MethodsPreparation of SC formulaTCM-SC formula was prepared as described previously.12 The ingredients of SC formula include salidro-side and curcumin, and the dose of SC formula was salidroside 5.77 mg/kg/d and curcumin 21.76 mg/kg/d, which were referred to the literature12 and based on the Pharmacopoeia of the People’s Republic of China. In our study, salidroside (98%, Batch No. ZL20160504HJT) and curcumin (98%, Batch No. ZL 20160309JHS) were purchased from Nanjing Zelang Medical Technology Co., Ltd. Rosiglitazone (Batch No.: 150109, Chengdu Hengrui Pharmaceutical Co., Ltd.) was applied as positive control for the treatment of NALFD. The usage dose of rosiglitazone for a 60-kg (body weight) adult was 4 mg/d. After calculation according to the conversion formula between humans and rats (1:6), a dose of 0.4 mg/kg/d was applied in rats, which was also referred to the literature.13
Animals and modeling28 SD rats (male, 100-120 g, clean grade) were purchased from Laboratory Animal Center of Zhejiang Academy of Medical Sciences (SCXK(Zhe)2014-0001) and raised in Animal Center of Ningbo University with free access to water.
The rats were randomly divided into control group (n = 7), NALFD model group (n = 7), SC treatment group (n = 7) and rosiglitazone treatment group (n = 7). NALFD was established by administration of high fat diet as previously described.13 Briefly, the NALFD rats were fed with high fat diet (10% lard, 2% Cholesterol and 88% basal diet) for 14 weeks. The high fat diet was purchased from Trophic Animal Feed High-Tech Co., Ltd., China. Control rats were fed with basal diet. From the ninth week after modeling, the rats in SC treatment, and rosiglitazone treatment groups received SC formula (27.53 mg/ kg/d) and rosiglitazone (0.4 mg/kg/d) for consecutive six weeks through intragavage administration, respectively, while the rats in control and model groups received similar volume of potable water.
Specimen collection14 weeks after modeling or administration, the rats were fasted for 12 h and anesthetized by 2% pentobarbital sodium (3 mL/kg, i.p.). Veinal blood was collected and liver tissues were isolated for hematoxylin-eosin staining (H&E) staining and biochemical detection.
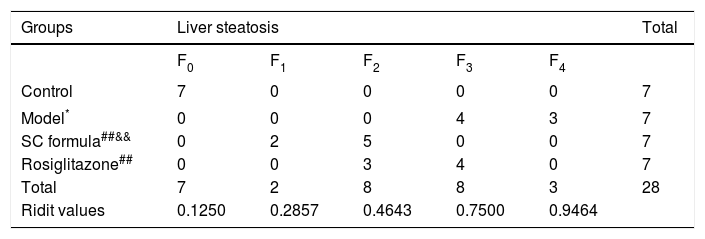
H&E stainingLiver tissues were fixed in 10% neutral formaldehyde solution. After fixation, the tissues were dehydrated by the automatic dehydration machine, and embedded in paraffin. The tissues were then sectioned into 4-μm thickness slices and underwent routine H&E staining. The slides were observed under light microscope. According to Nonalcoholic fatty liver disease diagnosis and treatment guidelines established by the Chinese Medical Association liver disease branch,14 the degree of liver steatosis is divided into five grades (F0–F4) as follows:
- •
F0, liver steatosis < 5%.
- •
F1, liver steatosis (5%–30%).
- •
F2, liver steatosis (31%–50%).
- •
F3, liver steatosis (51%–75%).
- •
F4, liver steatosis > 75%.
Liver triglyceride (TG) and free fatty acids (FFA) were detected as previously described.13 Sera alanine ami-notransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT) were detected as previously described.15
Fasting insulin (FINS) was detected as previously described.13 Fasting blood glucose test (FBG) was also detected. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated based on the formula: HOMA-IR = FBG x FINS/22.5.
Liver superoxide dismutase (SOD) (Batch No. 20170106) and malondialdehyde (MDA) (Batch No. 20170111) were detected by biochemical method according to the instructions of the kits as previously described.16
Enzyme-linked immunosorbent assay (ELISA)Sera tumor necrosis factor (TNF)-a and Interleukin (IL)-1 levels were detected by ELISA method following the instructions of the assay kits (Shanghai Yeyuan Biotechnology Company, Shanghai, China). Liver tissue AMP-activated protein kinase (AMPK), phosphorylated AMPK (pAMPK) malondialdehyde (MDA), acetyl-CoA carboxylase (ACCase), phosphorylated acetyl-CoA catboxylase (pACCase), carnitine palmitoyltransferase 1 (CPT-1), were also detected by ELISA method according to the instructions of the kits (Shanghai Yuanye Biotech, Shanghai, China). Liver tissue homogenate was prepared as previously described.15
Statistical analysisAll the data were in normal distribution, and expressed as mean and standard deviation, and analyzed by SPSS 16.0. The difference among groups was analyzed by ANOVA followed by LSD. P value less than 0.05 was considered as significant difference.
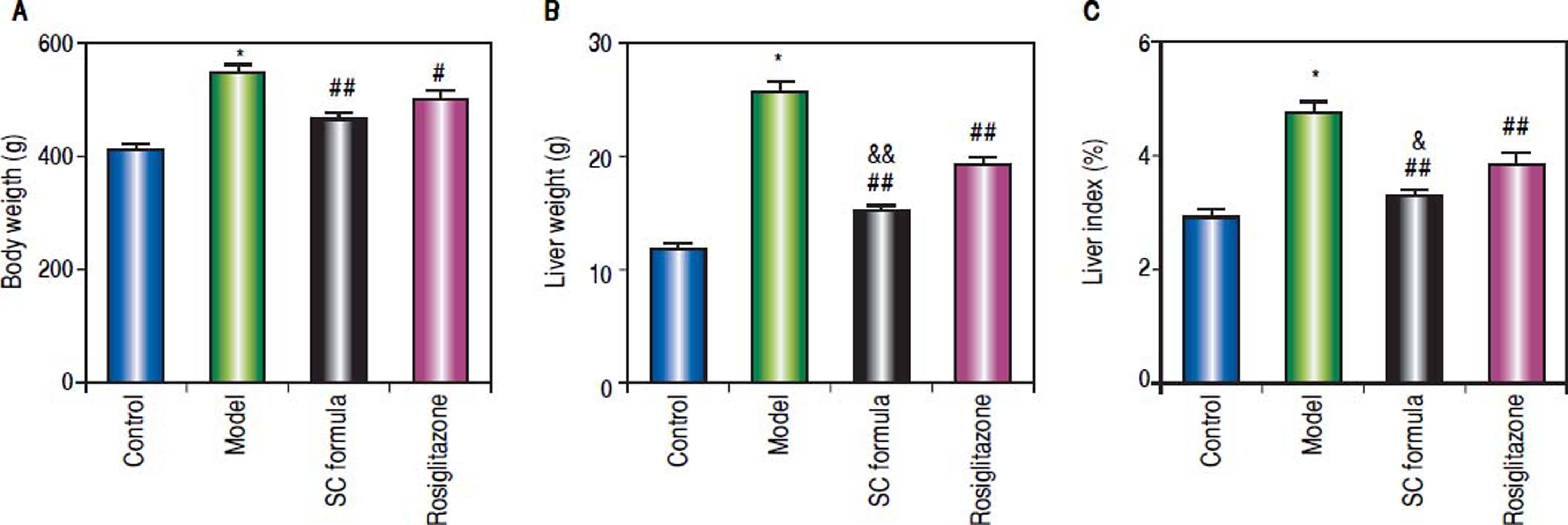
ResultsSC formula alleviates the high fat diet-induced liver injuryCompared with normal control, body weight, liver weight and liver index were significantly elevated in the rats from model group. By contrast, SC formula, as well as rosiglitazone significantly decreased high fat diet-induced increase of body weight, liver weight and liver index. Moreover, liver weight and index in SC formula treatment group were significantly lower than those in rosiglitazone group (Figure 1).
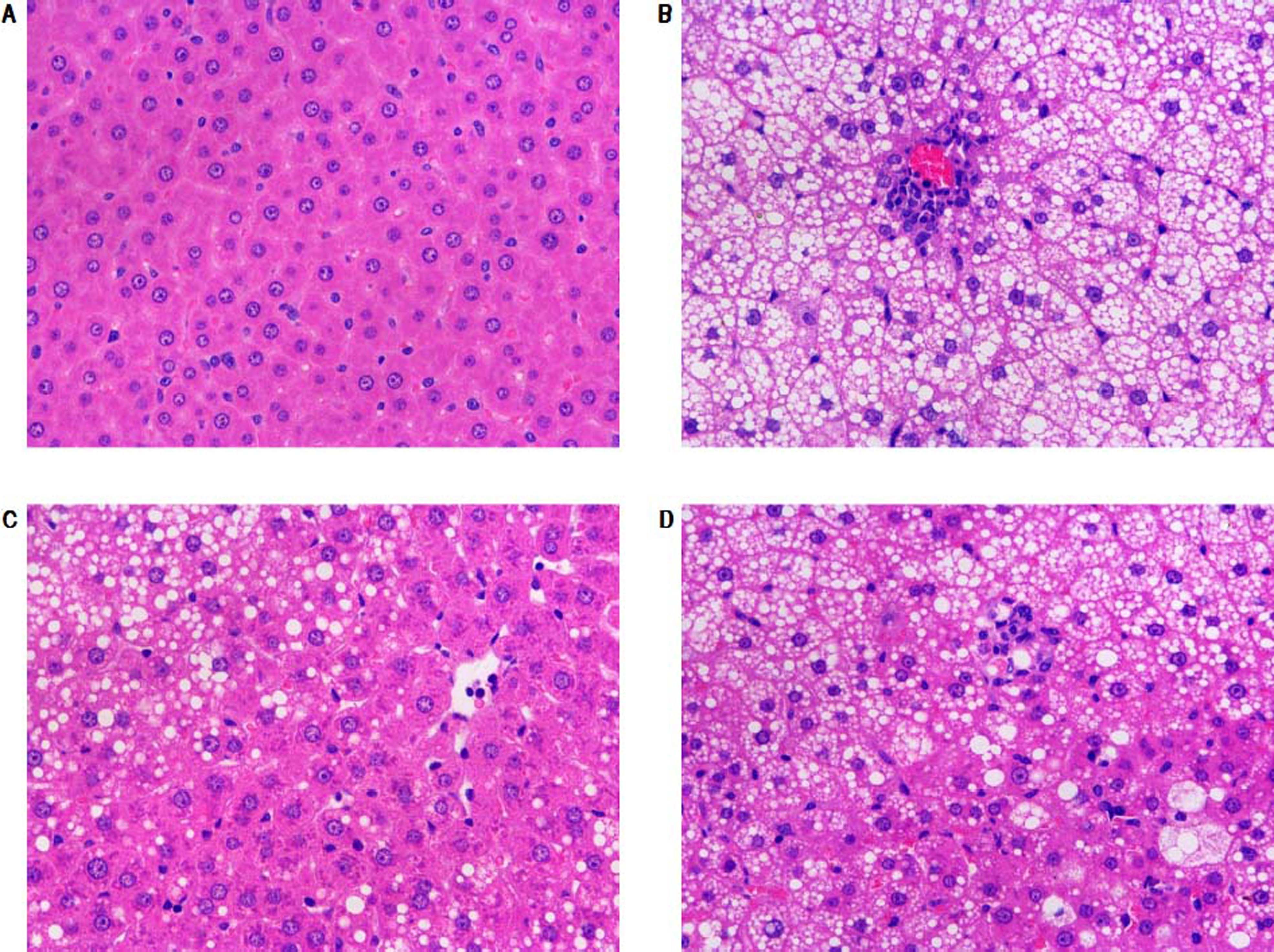
Morphological changes of liver tissue were also compared in different groups. As shown in figure 2, hepatic cells in control group had a normal morphologic. Lipid deposition was not observed in cytoplasm of the normal cells. There was no cell infiltration in the liver tissue (Figure 2). By contrast, lipid deposition was found in hepatic cells in model group. Nucleus shift was observed in some of the cells. Mild infiltration and necrosis were found in some of the hepatic cells. However, SC formula and rosiglitazone treatment remarkably mitigated high fat diet-caused liver injury. Additionally, the injury in SC formula was much milder, compared with rosiglitazone group. Liver steatosis was analyzed in different groups. As shown in table 1, liver steatosis was not observable in control group, while high steatosis was found in model group. SC formula and rosiglitazone treatment remarkably reduced high fat diet-caused liver steatosis. Additionally, the steatosis in SC formula was much milder, compared with rosiglitazone group.
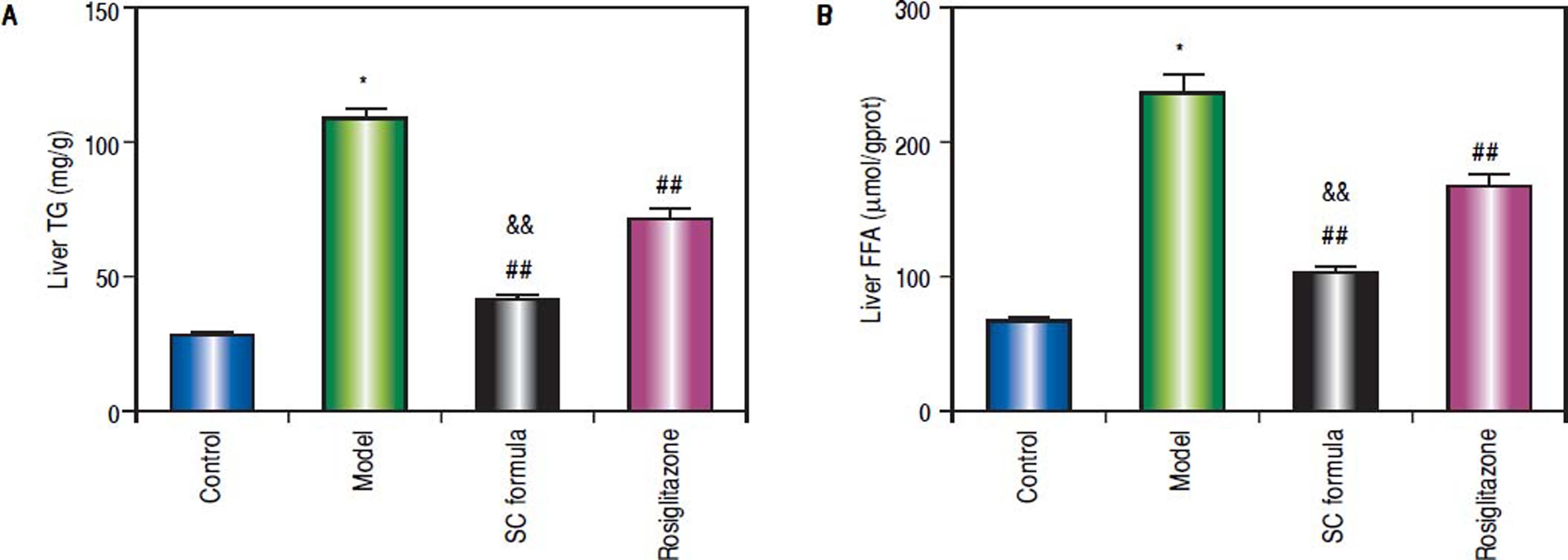
Compared with control, liver TG and FFA levels in model group were significantly elevated (Figure 3). SC formula, as well as rosiglitazone significantly decreased TG and FFA, compared with model group. Additionally, SC formula was more effective to alleviate TG and FFA compared with rosiglitazone group.
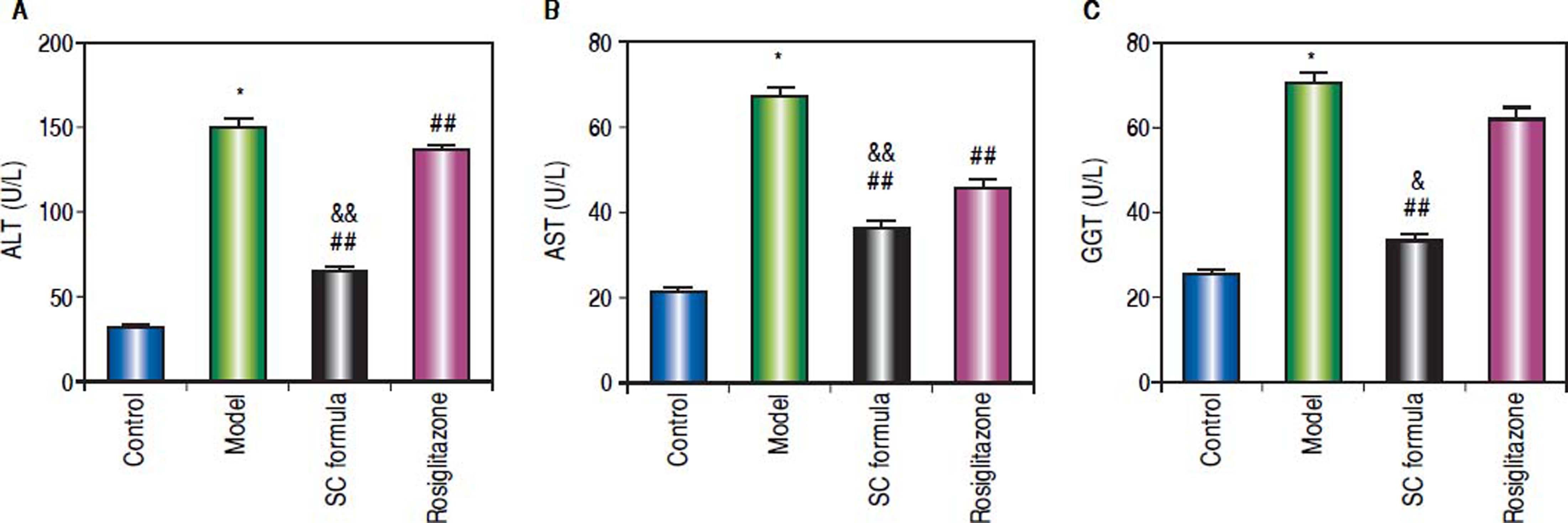
Effects of SC formula on sera ALT, AST and GGTThe liver function related enzymes, ALT, AST and GGT were detected. As compared to control, sera ALT, AST and GGT in model group were significantly elevated (Figure 4). By contrast, SC formula alleviated the high fat diet-induced increase of ALT, AST and GGT. By contrast, rosiglitazone alleviated ALT and AST, but not influenced GGT.
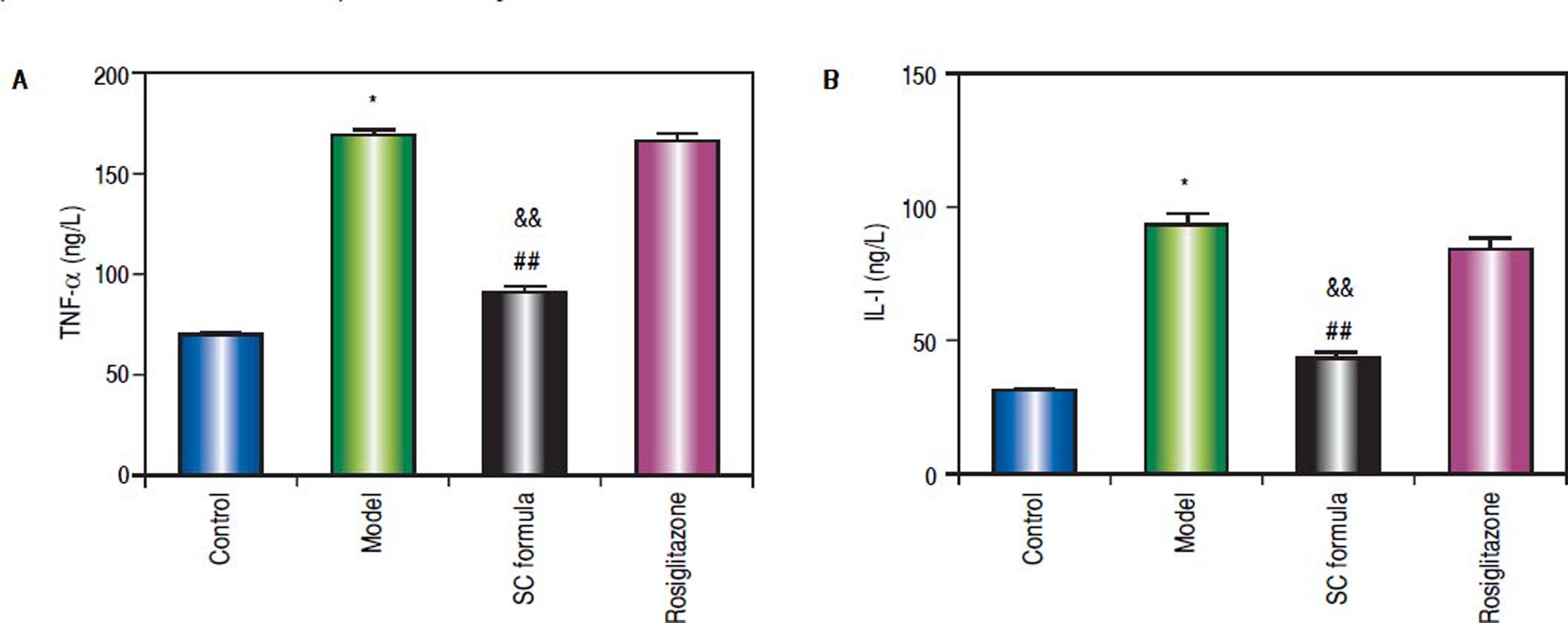
Effects of SC formula on sera TNF-α and IL-1The effects of SC formula on inflammation factors were also evaluated. As shown in figure 5, high fat diet promoted sera TNF-α and IL-1 levels. By contrast, SC formula reduced high fat diet-induced inflammation factors, while rosiglitazone did not influence TNF- α and IL-1 levels compared with model group.
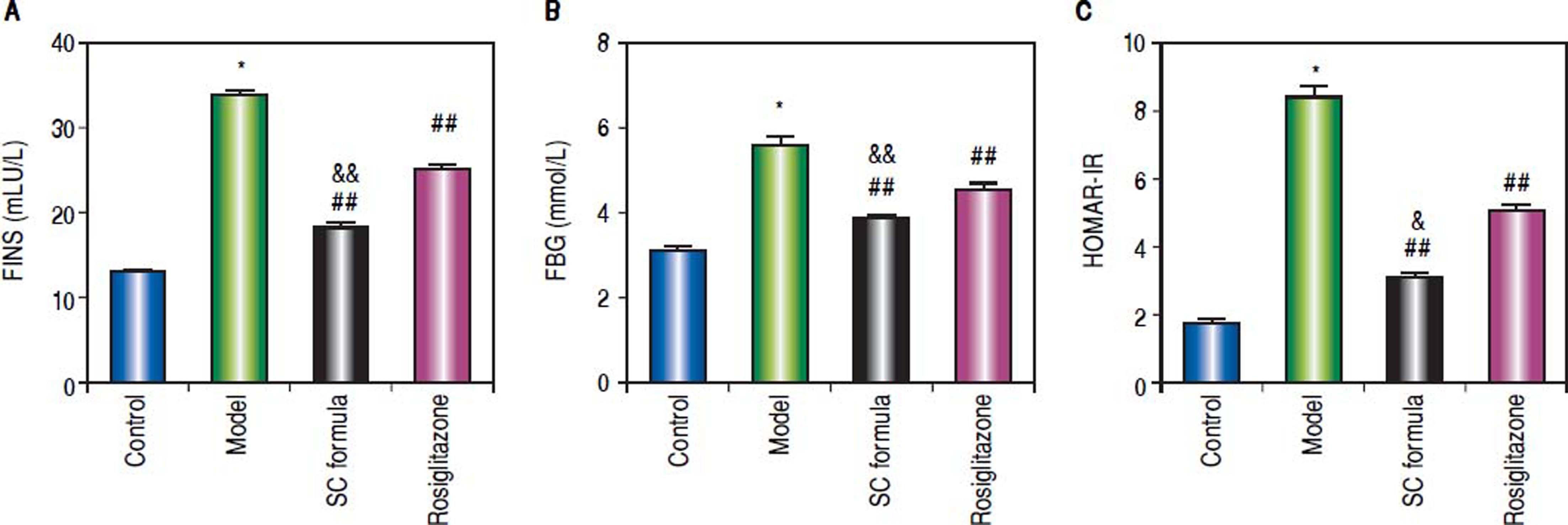
Effects of SC formula on sera FINS, FBG and HOMA-IRThe effects of SC formula on FINS, FBG and HOMAIR were also evaluated. As shown in figure 6, high fat diet elevated FINS, FBG and HOMA-IR levels, which were attenuated by SC formula, as well as by rosiglitazone. Additionally, SC formula was more effective to reduce FINS, FBG and HOMA-IR compared with rosiglitazone group.
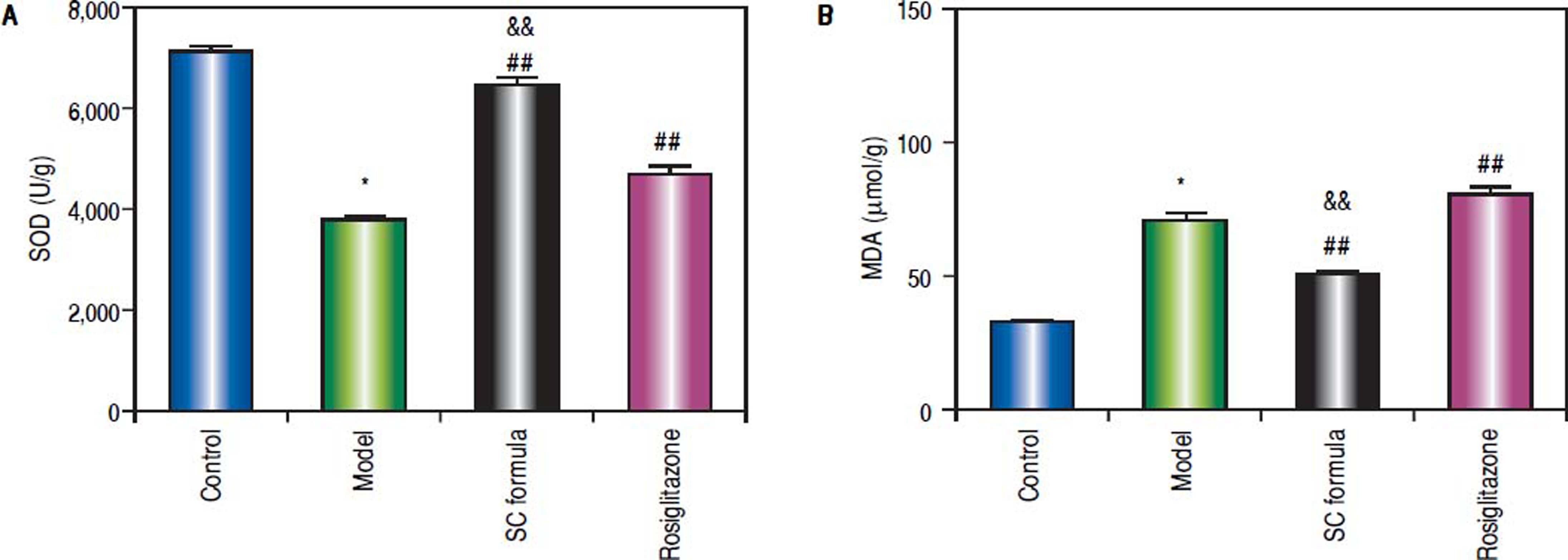
Effects of SC formula on tissue MDA and SODAs shown in figure 7, high fat diet decreased SOD and elevated MDA levels. By contrast, SC formula, as well as rosiglitazone alleviated the effects of high fat diet on MDA and SOD. Moreover, the effect of SC formula was prior to rosiglitazone.
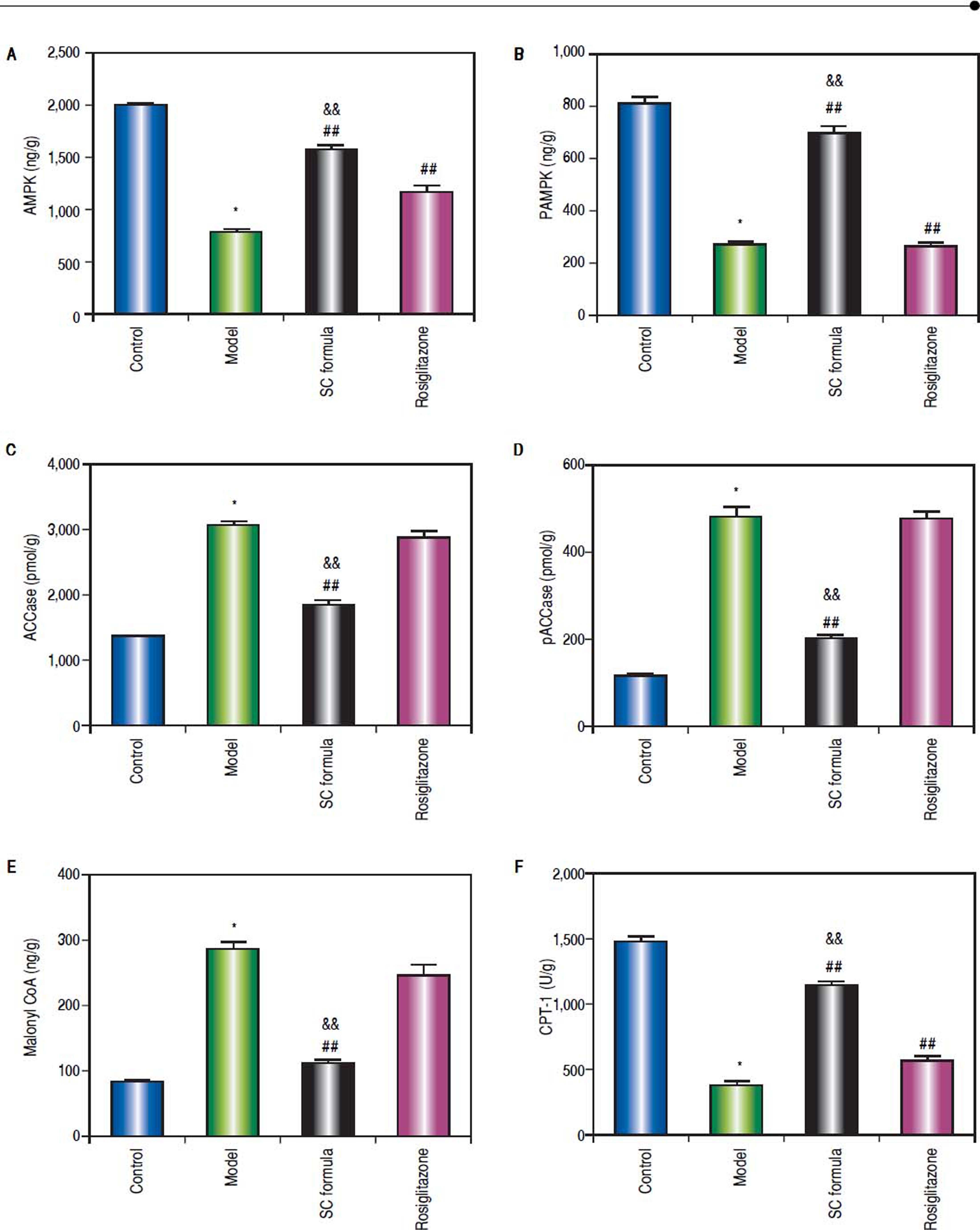
Effects of SC formula on tissue AMPK, pAMPK, ACCase, pACCase, Malonyl CoA and CPT-1We also detected AMPK signaling pathway in our study. As shown in figure 8, high fat diet decreased AMPK, pAMPK, and CPT-1, while increased ACCase, pACCase and Malonyl CoA. SC formula reduced the effect of high fat diet induced decrease of AMPK, pAMPK, and CPT-1, and increase of ACCase, pACCase and malonyl CoA, while osiglitazone only targeted AMPK and CPT-1. These data implicate that SC formula was prior to rosiglitazone to influence the AMPK signaling pathway.
DiscussionIn this study, we evidenced that SC formula alleviated high fat diet-induced liver injury. Besides its effects on liver morphological changes, SC formula also ameliorated liver function biochemically. Additionally, SC formula prevented inflammation reaction, enhanced anti-oxidative ability. The hepatoprotective effect of SC formula appeared to be mediated via its effects on the AMPK signaling pathway.
NAFLD shares a lot of common symptoms with obesity, dyslipidemia and diabetes.7,8 The common pathological changes include the disorders of glucose and lipid metabolism, and insulin resistance.9 Liver steatosis can also promote the development and progression of type 2 diabetes mellitus and atherosclerosis. Therefore, NAFLD is a complex and systemic metabolic disease, and it is difficult to design ideal drugs for single target. Treatment aimed at multiple targets or combination therapy has recently become the main stream to ameliorate the symptoms of NAFLD. For examples, vitamin E has the ability to decrease the activity of apoptosis-associated enzymes and has potential synergistic effects in combination with caspase (Caspase) inhibitors.17 Vit E and probiotics/prebiotics can synergistically increase their antioxidant stress and antiinflammatory efficacy.18 L-carnitine with antioxidant or L-carnitine with statins/fenofibrate can improve oxidative damage of mitochondrial (MT) and promote MT biogenesis.19 These studies provide certain reference to promote clinical and translational research of combination therapy for NAFLD.
In this study, we investigated the therapeutic effects of SC formula on NAFLD. SC formula is composed of salidroside and curcumin, two components obtained from TCM rhodiola rosea and rhizoma curcumae longae. A previous study has demonstrated that SC formula has good activity in inhibiting lipid deposition in the liver of NAFLD rats.12 We established NAFLD rat model by administrating high fat diet for 14 weeks. We further confirmed the efficacy of SC in the prevention and treatment of NAFLD. Liver TG and FFA were reduced after SC formula treatment. Additionally, sera ALT, AST, TNF-a, GGT activity and TNF-a, IL-1 content were alleviated in NAFLD after SC formula treatment.
The pathogenesis of NAFLD is still not clear, and the two “hits” theory proposed by Day et al has become a relatively recognized NAFLD pathogenesis.20 According to this theory, insulin resistance, formation of oxidative stress and antioxidant deficiencies play important roles in the pathogenesis of NAFLD. In our study, administration of high-fat diet induced insulin resistance, enhanced liver MDA content, and reduced liver SOD content in rats. These results suggest that NAFLD rats have obvious insulin resistance and oxidative stress. TCM SC formula can obviously reduce the insulin resistance and the content of MDA in liver tissue, and increase the content of SOD in liver tissue. These data implicate that SC formula has good effect on improving insulin resistance and lipid peroxidation injury.
As the key enzyme of fatty acid synthesis, ACCase plays a very important role in lipid metabolism. The activation of ACCase can increase the contents of Malonyl-CoA,21 which can inhibit the activity of CPT-1, thereby increasing high glucose/fatty acid induced islet glucolipotoxicity.22
AMPK is a key sensor of cellular energy status and a major regulator of lipid balance in the liver and whole body.23 It promotes energy metabolism by facilitating catabolism and reducing the consumption of adenosine triphosphate, thereby integrating nutrients and hormones signals. In the liver, activation of AMPK increases the oxidation of fatty acids and inhibits fatty acid synthesis. It can promote the inactivation and phosphorylation of acetyl coenzyme carboxylase, thus reducing the conversion of acetyl coenzyme A into C, thus reducing the synthesis of fatty acids.24 Our results showed that the activity of AMPK in liver tissue of model group decreased significantly and the contents of ACCase, pACCase and Malonyl-CoA increased significantly, and the content of CPT-1 in liver tissue decreased significantly. After SC formula treatment, the activity of AMPK in liver tissue was significantly increased, the contents of ACCase, pACCase and Malonyl-CoA in liver tissue were significantly decreased, and the content of CPT-1 in liver tissue was increased significantly. These data implicated that SC formula likely prevented liver injury through promoting AMPK signaling pathway.
Rosiglitazone was widely selected as a positive control for NAFLD treatment.25 In this study, we also selected rosiglitazone as a positive control. Consistent with previous publication,15 rosiglitazone repaired the liver injury in NAFLD model to some extent. Compared with SC formula, the efficacy was relatively low in the treatment. Rosiglitazone is a chemical compound, with the ability to activate peroxisome proliferator-activated receptor γ (PPARy). In our study, we evidenced that SC formula has a more extensive effects on inflammation reaction, insulin resistance and lipid oxidative stress. By contrast, rosiglitazone did not alter the inflammation reaction in NAFLD rats. Moreover, SC formula possesses stronger activity in fighting against insulin resistance and lipid oxidative stress. Additionally, the influence of SC formula on AMPK signaling pathway was more extensive compared with rosiglitazone. These factors together support SC formula as a prior candidate for treatment of NAFLD.
In our study, NAFLD rat model was selected to evaluate the protective activity of SC formula and assess the mechanisms. NAFLD model does not produce obvious hepatic fibrosis, but shares a lot of common features with obesity, dyslipidemia and diabetes. Although non-alcoholic steatohepatitis (NASH) is the severe form of NAFLD, NAFLD is the most common liver disorder in developed countries.26 Additionally, NAFLD without treatment could further develop into NASH.27 Therefore, it is likely preferable to control the disease at an early stage, when possible. In future study, NASH model will be established to further confirm the protective effect of SC formula and to detect the mechanisms.
ConclusionOur data reveal that SC formula prevents high fat diet-induced liver injury. Moreover, the efficacy of SC formula is prior to rosiglitazone. The potential mechanisms underlying the preventive effects were related to insulin resistance, lipid peroxidation and AMPK signaling pathway.
Abbreviations- •
ACCase: acetyl-coenzyme A carboxylase.
- •
ALT: alanine transaminase.
- •
AMPK: AMP-activated protein kinase.
- •
ANOVA: one-way analysis of variance.
- •
AST: aspartate aminotransferase.
- •
CPT-1: carnitine palmitoyl transferase-1.
- •
ELISA: enzyme-linked immunosorbent assay.
- •
FBG: fasting blood glucose.
- •
FFA: free fatty acid.
- •
FINS: fasting insulin.
- •
GGT: gamma-glutamyltransferase.
- •
H&E: hematoxylin and eosin.
- •
HOMA-IR: homeostasis model assessment of insulin.
- •
IL: interleukin.
- •
LSD: least significant difference test.
- •
malonyl CoA: malonyl coenzyme A.
- •
MDA: malondialdehyde.
- •
MT: mitochondrial.
- •
NAFLD: nonalcoholic fatty liver disease.
- •
NASH: nonalcoholic steatohepatitis.
- •
pACCase: phosphorylated acetyl-coenzyme A carboxylase.
- •
pAMPK: phosphorylated AMP-activated protein ki-nase.
- •
PPAR: peroxisome proliferator-activated receptor resistance.
- •
SC: salidroside and curcumin.
- •
SD: standard deviation.
- •
SOD: superoxide dismutase.
- •
TCM: Traditional Chinese Medicine.
- •
TG: triglyceride.
- •
TNF: sera tumor necrosis factor
- •
Vit E: vitamin E.
This study was supported by National Natural Science Foundation of China (81873109).
Conflict Of InterestThe authors declare that they have no conflicts of interest.