Background and rationale for the study. Hepatitis B virus (HBV) chronic infection may follow a benign course with low risk of cirrhosis or liver cancer. As differentiation of inactive status from HBeAg-negative chronic hepatitis B is often challenging, monitoring of inactive HBV carriers is important to detect viral relapse or formerly undetected activity. The incidence of hepatitis activity in HBeAg-negative carriers with normal aminotransferases was examined by retrospective analysis of a cohort of carriers who had been followed-up at a hospital in Central Brazil. All patients had remained free of evidence of liver disease and maintained normal aminotransferase levels throughout the first year of follow-up. The incidence density of chronic HBV activity was determined and an incidence curve was constructed using the Kaplan-Meier method. Cox regression models were developed to identify for surrogate markers of activity.
Results. Among the 224 patients who comprised the cohort, chronic HBV activity was detected in 30 during follow-up. The incidence density of activity was 11.8 per 100 person-years (95% confidence interval: 8.3-16.9). The results of Cox regression analysis indicated that chronic HBV activity was associated with entrance in the latter years of the period examined (p = 0.001) and initial normal aspartate aminotransferase (AST) levels close to the upper-normal value (p = 0.022).
Conclusion. Normal AST levels near the upper-normal value may be an indicator of relapse or previously undetected activity, and should thus be monitored closely in HBeAg-negative HBV carriers, in whom risk of relapse should remain an important managing consideration.
Infection with hepatitis B virus (HBV) is a major health problem worldwide, with more than 300 million people worldwide estimated to experience persistent HBV infection.1 Among chronic HBV infection carriers, those with continuous viral replication and liver necroinflamatory activity are at increased risk for developing cirrhosis and hepatocellular carcinoma (HCC).2 Many chronic HBV carriers eventually reach this chronic active phase of the disease, which reflects the effort of the immune system to clear the virus. Characterized by interruption of HBV replication and histological cessation of inflammation, this phase can be terminated by seroconversion of hepatitis B e antigen (HBeAg) to anti-HBe antibody.3,4 Patients who become inactive carriers are characterized by HBeAg negativity, presence of anti-HBe, low HBV DNA levels (< 2,000 IU/mL), and persistently normal alanine aminotransferase (ALT) levels.5,6 Generally, these patients have good outcomes and low risk of developing cirrhosis or liver cancer.3,7,8
Nevertheless, many inactive carriers can experience HBV replication and tissue inflammation reactivation. After a variable period of time throughout the development of precore or core promoter HBV mutant strains that prevent HBeAg production,9 these patients develop a form of HBV infection known as HBeAg-negative chronic hepatitis B. Patients in this phase of disease are usually older and at increased risk of developing the long-term complications of HBV infection, as are individuals who remain infected with the wild type virus (HBeAg-positive chronic hepatitis B). Recently, there has been increasing recognition that HBeAg-negative chronic hepatitis B is becoming more prevalent than the HBeAg-positive form in several countries, including Brazil.10,11
Currently one of the most important tasks of practitioners managing persistently HBV-infected patients is distinguishing patients with HBeAg-negative chronic hepatitis from inactive carriers. While the former requires treatment, the latter simply requires monitoring for viral relapse or inflammatory activity. Discriminating between these two groups of patients is challenging due to the frequent fluctuation of ALT and HBV DNA levels in HBeAg-negative chronic hepatitis patients.12 Hence, the main international guidelines on HBV management state that these patients cannot be classified as inactive carriers until confirmed as such by measurement of ALT and HBV DNA levels three or four times over the course of one year.6,13 To be classified as an inactive carrier, a patient’s ALT levels must remain under the upper normal limit and HBV DNA levels remain continuously below 2,000 IU/mL. After being classified as inactive carriers, patients must remain under lifelong monitoring to detect later relapse, a difficult task in areas of developing countries where molecular tests are often unavailable.
While most studies conducted in Western countries have found that inactive HBV carriers usually do not experience progressive disease and have good long-term prognosis,3,14 several have reported non-negligible rates of relapse.15,16 This variability is not completely understood, but may be dependent on the HBV DNA level of replication used to determine activity or the life period in which HBV infection was acquired. Despite the need to identify the factors in this variability, no studies, to our knowledge, have examined the natural history of chronic HBsAg-positive/HBeAg-negative carriers in Brazil. To fill this research gap, this study examined the course of infection in a cohort of HBeAg-negative carriers with normal ALT status in Central Brazil to elucidate the risk of progression to active chronic hepatitis.
Material and MethodsThis retrospective cohort study aimed to assess the incidence of HBV relapse or previously undetected activity in HBeAg-negative HBV carriers with normal aminotransferase levels. Data were collected from the medical files of patients followed-up at the Reference Liver Outpatient Unit of the Julio Muller Hospital (Mato Grosso Federal University), in Cuiabá, the main city of the region. Mato Grosso State is a vast region that corresponds to 10% of Brazilian territory and comprises part of the southern Amazon basin (Figure 1). In this region, the large distances and the irregular quality of public health procedures limit access to laboratory and medical facilities, mainly among low-income individuals. Few patients residing in this region have access to molecular testing of HBV DNA levels due to the high cost and lack of trained personnel. In the past, a few patients have paid to undergo molecular testing at private institutions. In the latest years, the access to this test has gradually improved due to Brazilian public health effort. Data were collected from the medical records of patients who initiated follow-up between 1997 and 2011 and had undergone follow-up for at least one year. The inclusion criteria were designed to select HBsAg-positive, HBeAg-negative patients with a normal range of aminotransferase levels and no evidence of chronic liver disease, as assessed by prothrombin time (≥ 85%), bilirubin level (≤ 1.0 mg/dL), albumin level ( ≥ 3.5 g/dL), platelet count (≥ 150,000 cells/mm3) and5 normal upper abdominal echography. The exclusion criteria were:
- •
Detection of more than 2,000 IU/mL or 10,000 copies/mL of HBV DNA if the patient had undergone polymerase chain reaction quantitation assay.
- •
Observation of several criteria that suggested HBV replication or active liver disease during the first year of monitoring, a criterion that aimed to ensure that all patients conformed to the international concept of an inactive carrier.
- •
Evidence of any other chronic liver disease, such as autoimmune hepatitis, hemochromatosis, Wilson disease, or non-alcoholic steatohepatitis.
- •
Coinfection with hepatitis C or D virus or HIV; and/or
- •
Daily alcohol consumption of more than 20 g.
The collected data were entered into the Microsoft Excel 2010 software for analysis using the appropriate statistical tests for comparison of continuous and categoric variables within a respective dispersion and confidence interval of 95% (95% CI). After performance of the Shapiro-Wilk test for continuous variables to verify normality, variables found to have non-parametric distribution were subjected to logarithmic, inverse, or quadratic transformation to attempt their normalization. Those variables that retained non-parametric distribution were described in terms of median values and analyzed by the Mann-Whitney U-test.
The outcome of the study was any evidence of HBV replication, liver inflammatory activity, or liver disease progression as defined by any one of the following criteria:
- •
ALT above the upper normal level determined according each fabricant specifications.
- •
HBV DNA level > 2,000 IU/mL or 10,000 copies/ mL, and
- •
Signs of cirrhosis or hepatocellular carcinoma.
The individual time of follow-up was computed from the first visit to loss of follow-up or the last visit (the day of censure). Data were collected until February 2013. For the analysis of the cohort, probability curves for hepatitis relapse over time were created using the Kaplan-Meier method. Cox’s logistic models were created to identify variables that may have been surrogate markers of previously undetected activity or forthcoming relapse. Baseline laboratory measures, sex, and initial age were included in the models and adjusted in a manner that allowed for identification of trends regarding relapse or previously undetected activity. The year of cohort entrance was also included to attempt to adjust for improvement in follow-up quality. Hazard risk (HR) was calculated within its respective 95% CI using Stata® 8.2 software (Statacorp, College Station, TX, USA).
Permission to conduct the study was obtained from the medical director of the hospital at which it was performed. The study was registered with the National System for Ethical Analysis (Plataforma Brasil-CONEP) with the protocol code 02102012. 0.0000.5163 and approved by the Hospital Ethical Board for Research on Human Beings on August 23, 2012.
ResultsReview of more than 2,500 medical files from the Reference Liver Outpatient Unit allowed for identification of 224 patients who met all the inclusion criteria and none of the exclusion criteria. Although the intervals between the patients’ visits ranged widely, HBeAg-negative carriers with normal aminotransferase levels had been routinely scheduled for a visit every six months after the first year by the Reference Liver Outpatient Unit. Males comprised 58.9% of the patients, among whom the median age at first visit was 39 years (interquartile range: 33-50 years). The year of entrance in the cohort ranged from 1997 to 2011, but most (77.2%) patients had initiated follow-up in 2005 or later. The median duration of follow-up, which included the first 12 months of observation without evidence of reactivation, was 19 months (interquartile range: 15-24 months). Of the 88 (39.3%) patients who had undergone HBV DNA quantitation before entrance into the cohort, 73 (83%) initiated follow-up in the last four years of the study, with 2008 their median year of entrance compared to 2006 for the entire cohort (p = 0.145).
Table 1 shows the cohort’s basal laboratory characteristics at entrance. Aspartate aminotransferase (AST) and ALT levels are shown as ratios between the values measured and the upper normal limits to attempt to standardize the values obtained by tests performed by different labs over a large time interval. As can be observed, 30 patients exhibited some evidence of liver disease activity, including 22 with ALT levels above the upper normal limit, 12 with HBV DNA levels above 2,000 IU/mL, and three with diagnosis of cirrhosis (one of these developed a HCC). Among the 12 with HBV DNA levels above 2,000 IU/mL, ten had DNA levels between 2,000 and 20,000 IU/mL and the other two had 38,000 and 1,000,000 IU/mL.
Baseline characteristics of cohort of HBeAg-negative HBV carriers with normal aminotransferase levels, Mato Grosso, Brazil.
| Characteristic | |
|---|---|
| Sex, n (%) | |
| Male | 132 (58.9) |
| Female | 92 (41.1) |
| Age at study entrance (years), n (%) | |
| ≤ 20 | 6 (2.7) |
| 21-30 | 28 (12.5) |
| 31-40 | 84 (37.5) |
| 41-50 | 52 (23.2) |
| 51-60 | 34 (15.2) |
| 61-70 | 14 (6.2) |
| ≥ 71 | 6 (2.7) |
| Duration of follow-up (months), n (%) | |
| 12-24 | 173 (77.2) |
| 25-36 | 26 (11.6) |
| 37-48 | 5 (2.2) |
| 49-60 | 6 (2.7) |
| ≥ 61 | 14 (6.3) |
| Year of entrance into cohort, n (%) | |
| 2003 or earlier | 33 (14.7) |
| 2004-2005 | 47 (21.0) |
| 2006-2007 | 66 (29.5) |
| 2008-2009 | 51 (22.8) |
| 2010-2011 | 27 (12.0) |
| Albumin level (g/dL)* | 4.1 (3.5-4.5) |
| Platelet count (×103)* | 214 (154-326) |
| Prothrombin time (% activity)* | 95 (74-100) |
| AST ratiot†,* | 0.58 (0.22-0.98) |
| ALT ratiot†,* | 0.64 (0.27-0.98) |
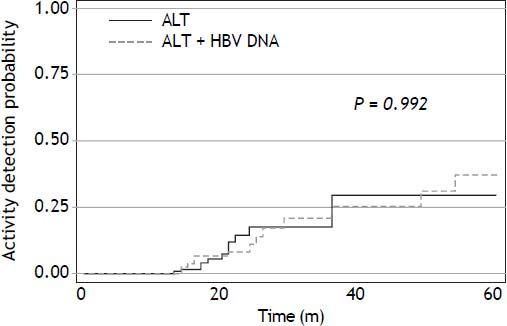
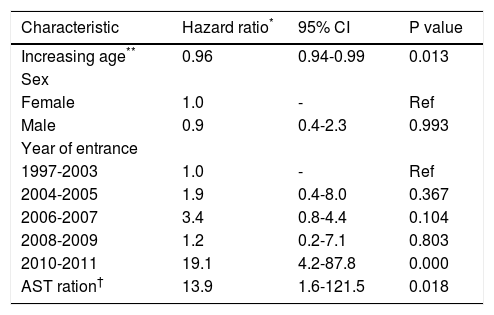
Figure 2 shows the estimated chronic HBV activity detection as a function of time and table 2 the incidence density of activity detection by year. A large number of patients were censured due to missing follow-up data producing inaccurate estimation in the last years of follow-up. As can be observed, the overall incidence density was 11.8 events per 100 persons at risk per year and the incidence density was relatively steady in the first years of the follow-up, ranging from 10.5 and 12.5 events per 100 persons at risk per year. No difference in risk was found between those who had undergone HBV DNA measurement and those who had not (log-rank χ2 p-value = 0.470; Figure 3). Patients for whom activity was detected had been followed-up for a longer duration than patients for whom it was not (median duration: 23 vs. 18 months, p = 0.003). For example, the three patients who developed cirrhosis were older (48, 51, and 64 years) and were followed-up for longer (73, 69, and 86 months) than most of the cohort members. Multivariate analysis revealed that having entered the cohort at the latter years and AST ratio were associated with outcome in all the models created, as well as a lower risk of activity detection in relatively older HBV carriers. Table 3 shows the model found to best fit the data (log-rank χ2 = 33.8).
Incidence density of reactivation by year in a cohort of HBeAg-negative HBV carriers with normal aminotransferase levels in Mato Grosso, Brazil.
| Year of the cohort | Person-years followed up | Failures (n) | Incidence density^ | Incidence density 95% CI† |
|---|---|---|---|---|
| 1 | 224 | 0 | 0 | - |
| 2 | 143 | 15 | 10.5 | 6.3-17.4 |
| 3 | 40.7 | 5 | 12.3 | 5.1-29.7 |
| 4 | 24.7 | 3 | 12.2 | 3.9-37.9 |
| 5 | 18 | 2 | 11.1 | 2.8-44.6 |
| 6 | 12.4 | 2 | 16.1 | 3.9-64.4 |
| 7 | 6.7 | 1 | 15.0 | 2.0-166.4 |
| 8 | 3.7 | 1 | 26.7 | 3.7-189.7 |
| 9-10 | 3.9 | 1 | 25.5 | 3.5-181.6 |
| Total | 253* | 30 | 11.8 | 8.3-16.9 |
Probability curve for detection of chronic hepatitis B activity as a function of time, stratified by whether HBV DNA monitoring was conducted (dashed line) or not (solid line). Log-rank χ2 p-value of the first five years of follow-up is displayed in the figure. Log-rank χ2 p-value of the overall cohort: 0.470.
Cox regression analysis to assess risk of chronic hepatitis B activity in HBeAg-negative HBV-carriers with normal aminotransferase levels, Mato Grosso, Brazil.
| Characteristic | Hazard ratio* | 95% CI | P value |
|---|---|---|---|
| Increasing age** | 0.96 | 0.94-0.99 | 0.013 |
| Sex | |||
| Female | 1.0 | - | Ref |
| Male | 0.9 | 0.4-2.3 | 0.993 |
| Year of entrance | |||
| 1997-2003 | 1.0 | - | Ref |
| 2004-2005 | 1.9 | 0.4-8.0 | 0.367 |
| 2006-2007 | 3.4 | 0.8-4.4 | 0.104 |
| 2008-2009 | 1.2 | 0.2-7.1 | 0.803 |
| 2010-2011 | 19.1 | 4.2-87.8 | 0.000 |
| AST ration† | 13.9 | 1.6-121.5 | 0.018 |
Most previous studies of low-active chronic HBV carriers reported a benign prognosis and low risk of complications.3,14 Detection of chronic HBV complications in these patients was generally highest in Eastern countries and lowest in Western countries.7,14,16–18 Among them, Chu and Liaw15 reported a HBV relapse incidence of 10% in Chinese inactive carriers followed for five years. In contrast, Manno, et al.7 reported liver disease in less than 2% of carriers after 30 years in northern Italy, and Villeneuve, et al.14 reported a similar incidence in Montreal. While the differences among these and the findings of other studies may be accounted for by differences in demographic factors, genetic factors of the host or virus, or the outcomes analyzed, they can be chiefly attributed to the use of different definitions of an inactive carrier.
The present study attempted to fill the research gap regarding the natural course of inactive HBV carriers in Latin America by analysis of the detection rate of HBV relapse or formerly unidentified activity in a cohort of HBeAg-negative HBV carriers with normal aminotransferase levels in Brazil. As HBV DNA monitoring was not possible for most of the patients examined, they could not be categorized as inactive HBV carriers. The cohort thus likely consisted of both inactive carriers and low-activity chronic hepatitis B carriers, and was the reason for the higher detection rate of previously unrecognized activity or relapse (11.8 events per 100 persons at risk per year) compared to other Western studies.14,19 The setting of limited available resources described here is likely typical of large areas of Brazil and South America, although no other Brazilian studies addressing this issue could be found in the main biomedical databases. As molecular testing had been performed for less than half of the subjects, most carriers with disease activity were identified by detection of ALT elevation. Between the second and the fifth years of follow-up, the incidence of activity was between 10 and 12 events per person-year. After the fifth year of follow-up, the number of cohort participants significantly decreased, which precluded the drawing of any conclusions from the findings. However, the findings from the initial years support that biochemical monitoring of AST and ALT levels remains useful in the detection of disease activity, especially in regions where molecular testing is not readily available.
Although all patients had initial aminotransferase levels in the normal range, those with AST values closer to upper normal limits had a higher risk of disease activity detection during follow-up. In recent years, liver disease experts have warned that the normal limits for aminotransferase levels should be lowered, especially due to the development of fatty liver disease in obese patients.20 This finding seems to corroborate the necessity to be more restrictive regarding the setting of upper-normal aminotransferase levels. Curiously, this association was not observed regarding ALT elevation, which is considered a more specific means of detecting liver injury than AST elevation. Nevertheless, the possibility that the association between disease activity and an upper-normal AST level was false cannot be eliminated, as the presence of an unrecognized confounder may have affected the findings. Until clarification of this association by the performance of additional studies, HBV activity should be suspected even in patients with normal AST but close to the upper limit.
The HBV DNA level used in Brazil to define HBV activity is currently 2,000 IU/mL (Brazilian Health Ministry determination since October 2009), a cutoff level that appears stringent compared to those used in the past. In fact, some researchers use 20,000 IU/ mL as the limit for defining HBV activity.18,19 In the cohort examined in the present study, HBV DNA levels did not exceed 20,000 IU/mL in 10 of the 12 patients in whom HBV activity was detected by measurement of increased HBV DNA levels, and ALT elevation was not detected in six. While some of these patients may have continued to experience a less aggressive form of disease and low risk of complications, current Brazilian public guidelines specify that liver biopsy is optional and that nucleos(t)ide analogue therapy should be initiated for them. An association between younger age and risk of disease activity emerged after adjustment by Cox regression. This association may be due to a survival bias in that older patients with active hepatitis had a greater probability of earlier detection, and thus would be already living for a longer duration with a low level of HBV replication.
The present study faced several limitations that should be considered when reviewing the findings. One limitation was that it was unable to explore long-term outcomes, such as cirrhosis or HCC development, as most patients were followed for less than two years, a period insufficient to allow the occurrence of such outcomes. Nevertheless, disease progression was detected in three patients via new diagnosis of cirrhosis, who were among those who were followed-up for more than five years. This finding also suggests failure in the initial classification of at least some patients. More strategies to screen liver health status of these carriers such as liver elastography would be suitable. Another limitation was the lack of molecular testing to detect HBV DNA in most of the patients, which likely led to incidence underestimation.21 Nonetheless, the HBV relapse rates for those who had undergone HBV DNA testing and those who had not were similar. The increased detection rate in the two most recent years of the cohort, as identified in the adjusted analysis, may indicate that the most recently available PCR tests have allowed for improved quality of care of chronic HBV carriers. Detection improvement may also have occurred due to a positive learning curve among medical staff. A final limitation was the loss of a significant number of patients to follow-up, especially after the first two years. In consideration of this limitation, data collected during the latter cohort years and the right side of the Kaplan-Meier curves should be interpreted cautiously.
This retrospective analysis of a cohort of HBeAg-negative HBV-infected patients to differentiate true inactive carriers from those with chronic HBV activity revealed a non-negligible rate of HBV activity detection. This finding indicates the need to remain aware of the risk of relapse and undetected activity in this patient population. The findings also indicate that an initial normal AST level near the upper-normal value may be an indicator of HBV activity. Further studies should now attempt to clarify the relationship between AST level and HBV activity in inactive carriers. Public health authorities and practitioners at sites facing resource limitations similar to those in the setting examined here should consider these findings when treating chronic HBV-infected and HBeAg-negative patients.
Abbreviations- •
95% CI: confidence interval of 95%.
- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
HBeAg: hepatitis B e antigen.
- •
HBV: hepatitis B virus.
- •
HBV DNA: polymerase chain reaction for detection of hepatitis B virus DNA.
- •
HCC: hepatocellular carcinoma.
- •
HIV: human immunodeficiency virus.
- •
HR: hazard risk.
Souto FJ receives a regular support grant from CNPq (Brazilian Agency for the Scientific and Technological Development).