Background and rational. Telaprevir-based therapy (TBT) has been extensively evaluated in clinical trials. So we designed a study to compare the efficacy and safety of TBT between patients with moderate fibrosis and those suffering from advanced fibrosis in clinical practice. A multicenter observational and ambispective study was conducted. It included 582 patients with chronic hepatitis C genotype 1, 214 with fibrosis F2, and 368 with F3/F4 (F3: 148; F4: 220).
Results. The mean patient age was 55 years, 67% male. Type of prior response was 22% naïve, 57% relapsers, and 21% partial/null responders, 69% had high viral load (> 800,000 IU/mL). HCV genotypes were 1a (19%), 1b (69%), and 1 (12%), respectively. Sixty-five percent were non-CC IL28B genotype. Week-12 sustained virologic response (SVR12) was significantly higher among F2-naïve patients (78%) compared with F3/F4-naïve patients (60%; p = 0.039) and among F2 non-responders (67%) compared with F3/F4 non-responders (42%; p = 0.014). SVR12 among relapsers was remarkably high in both groups (F2:89% vs. F3/F4:78%). Severe anemia and thrombocytopenia were more frequent among patients with F3/F4 than those with F2 (p < 0.01). Overall, 132 patients (22%) discontinued treatment: 58 due to adverse effects, 42 due to the stopping-rule, and 32 due to breakthrough. Premature discontinuation was more frequent among patients with F3/F4 (p = 0.028), especially due to breakthrough (p < 0.001). Conclusions. This multicenter study demonstrates high efficacy and an acceptable safety profile with regard to TBT in F2-patients in clinical practice.
Only between 15 and 45% of patients infected with hepatitis C virus (HCV) spontaneously clear the infection. The remaining patients develop chronic infections with a risk of cirrhosis development in the long term between 15 and 30%. It is estimated that HCV is involved in 28% of cases of cirrhosis and 26% of the cases of liver cancer worldwide, representing approximately 500,000 deaths per year.1 The risk of progression to cirrhosis is variable and influenced by various factors such as alcohol consumption, age of infection, fibrosis, viral genotype, co-infection with HIV or HBV, and other comorbidities.2 HCV liver disease and its complications are the leading cause of liver transplantation in most European countries.3
The treatment goal of chronic hepatitis C (CHC) is to achieve virological cure. The approval of the first-generation protease inhibitors in 2011, boceprevir and telaprevir, marked the beginning of a new era in the treatment of CHC. Phase III studies have shown that triple therapy can achieve sustained virologic response (SVR) in approximately 67-75% of naïve patients and in more than 80% of relapsers4–6 infected with genotype 1. These results significantly improved treatment efficacy based on pegylated-interferon and ribavirin(PR).4–6 Recent advances have been impressive, including the development of new direct antiviral agents (DAAs) that offer high SVR rates with minimal adverse effects.7–9 In fact, the December 2013 guidelines of AASLD suggest that therapy with telaprevir or boceprevir should not be indicated at this time.10 More recently, during the last congress of the EASL in London, both the EASL and the World Health Organization (WHO) developed guidelines that list the most relevant aspects of the current situation of this infection and its treatment. In addition, these guidelines11 allow for recommendations based on the social and political realities in which, the vast majority of patients infected with HCV, live. In this sense, these guidelines explicitly consider the need of telaprevir or boceprevir-based therapy in situations in which other treatment options are not possible.
While newer therapies will likely lower the barriers to CHC treatment because of ease of administration, short therapy duration, excellent patient tolerability, and limited drug-interactions, not all barriers to treatment initiation will disappear. Indeed, the high cost of this therapy will at least temporarily limit its access in some countries.12–14 In fact, we recently showed that the restrictions imposed by health services are one of the major barriers to treatment initiation, and these limitations have occurred with drugs that clearly cost less than sofosbuvir.15 Finally, the introduction of new drugs in clinical practice is a slow process, from authorization to their actual availability that delays the arrival of these drugs for certain patients.
For these reasons, we studied actual clinical practice to determine the current role of TBT among patients with hepatitis C genotype 1 at a time when treatment with a new DAA was being adopted in Spain. The primary objective of this study was to compare the efficacy and safety of TBT in routine clinical practice among large cohorts of patients with either moderate fibrosis (F2) or advanced fibrosis (F3/F4).
Material and MethodsA multicenter observational and ambispective study was conducted. The target population were patients with CHC genotype 1 treated with TBT. Patients from 23 Spanish hospitals were included. Enrollment began on November 1, 2012 to November 1, 2013. The protocol was evaluated and approved by the Spanish Agency for Medicines and Medical Devices (Protocol code JCG-TEL-2013-01).
Inclusion criteria were:
- •
Age above 18 years.
- •
Diagnosed with CHC genotype 1 and either F2 or F3/F4 treated with TBT.
- •
Daily alcohol consumption < 40 g.
- •
Any type of previous response profile to PR: naïve or non-responders (relapsers, partial, null-responders).
- •
Fibrosis evaluation: biopsy or transient elastography (the most recent determination) categorized using the METAVIR scale.
Patients were excluded if they had:
- •
Co-infections (HBV, HDV, HIV).
- •
Hepatocellular carcinoma.
- •
Renal failure (creatinine clearance < 50 mL/ min); or
- •
Solid organ transplants.
TBT management at each site followed the product data sheet and the recommendations established by the Spanish Agency for Medicines and Medical Devices.
Primary objectivesThe current study compared the efficacy and safety of TBT among patients with F2 and in those among patients with F3/F4. Treatment efficacy was established by analyzing SVR at 12 weeks after treatment completion. Viral load determination was performed using m2000SP/m2000RT (Abbott Molecular, Des Moines, IL) and COBAS AmpliPrep®/COBAS TaqMan (Roche Molecular Systems, Pleasanton, CA) in the center from which the patient was referred.
SafetyAdverse events (AE) were recorded for all patients during treatment and 12 weeks after its completion. AE grade 3-4, serious adverse effects (with special attention to anemia beyond grade 2), infections, hepatic decompensation, and other cytopenias were included. Management of anemia was at investigator’s criteria.
Ethics and confidentialityThose responsible for registration procedures and researchers agreed to follow the applicable ethical and legal standards, particularly the Declaration of Helsinki, the Oviedo Convention, and the rules of good practice with regard to human-participants research. The respective Clinical Research Ethics Committees of all participating hospitals approved the current study. Study participants were all informed of the study’s objectives and an appropriate informed consent was obtained.
Statistical analysesThe descriptive analyses of the qualitative variables were performed by obtaining frequencies; those of the quantitative variables were performed using means and standard deviations or medians and range or interquartile deviation based on the presence of a normal distribution according to the Kolmogorov-Smirnov test. Univariate analyses for the qualitative variables were performed using the chisquare test. Student’s i-test was performed for quantitative variables. In other cases, an ANOVA was performed if the quantitative variable was normal, and the non-parametric Mann-Whitney and Kruskal-Wallis tests were used for non-normally distributed quantitative variables; CI95% were determined. Multivariate analyses were performed using a logistic regression model that included the SVR binary variable (yes/no) as the dependent variable and the covariates of interest as the independent variables. The covariates of interest for inclusion in multivariate analysis were selected based on statistical significance in the univariate analysis, clinical significance, or both. AEs occurred in both groups were tabulated to perform a descriptive analysis. The magnitude of the effect was described using odds ratios and CI95%. The significance threshold was set at p < 0.05. All analyses were performed using SPSS version 20.0 (IBM).
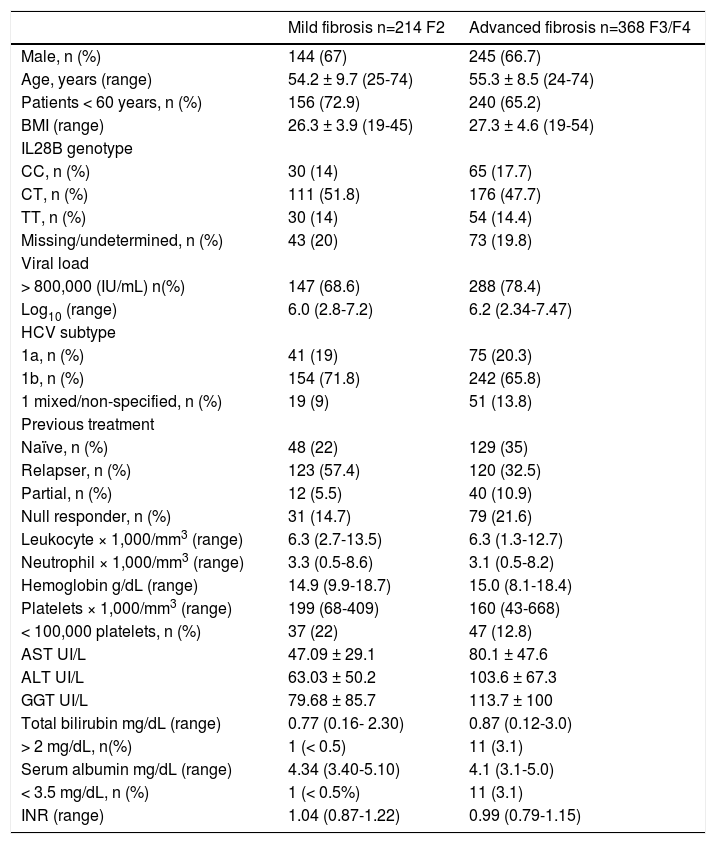
ResultsPatient characteristicsThe current study included 582 patients from 23 hospitals in Spain. These patients were categorized into two groups according to fibrosis level at baseline: 214 patients with F2 and 368 patients with F3/ F4 (148 patients F3 and 220 patients F4). Table 1 describes baseline characteristics of both groups. The majority were male (67%) with a mean age of 55 years old (70% of patients were younger than 60 years old). A clear predominance of unfavorable IL28B-genotype was observed. Over 70% of patients were infected with HCV genotype 1b and had high viral load (HCV-RNA > 800,000 IU/mL) at baseline.
Demographic, baseline patient characteristics and biochemical parameters.
| Mild fibrosis n=214 F2 | Advanced fibrosis n=368 F3/F4 | |
|---|---|---|
| Male, n (%) | 144 (67) | 245 (66.7) |
| Age, years (range) | 54.2 ± 9.7 (25-74) | 55.3 ± 8.5 (24-74) |
| Patients < 60 years, n (%) | 156 (72.9) | 240 (65.2) |
| BMI (range) | 26.3 ± 3.9 (19-45) | 27.3 ± 4.6 (19-54) |
| IL28B genotype | ||
| CC, n (%) | 30 (14) | 65 (17.7) |
| CT, n (%) | 111 (51.8) | 176 (47.7) |
| TT, n (%) | 30 (14) | 54 (14.4) |
| Missing/undetermined, n (%) | 43 (20) | 73 (19.8) |
| Viral load | ||
| > 800,000 (IU/mL) n(%) | 147 (68.6) | 288 (78.4) |
| Log10 (range) | 6.0 (2.8-7.2) | 6.2 (2.34-7.47) |
| HCV subtype | ||
| 1a, n (%) | 41 (19) | 75 (20.3) |
| 1b, n (%) | 154 (71.8) | 242 (65.8) |
| 1 mixed/non-specified, n (%) | 19 (9) | 51 (13.8) |
| Previous treatment | ||
| Naïve, n (%) | 48 (22) | 129 (35) |
| Relapser, n (%) | 123 (57.4) | 120 (32.5) |
| Partial, n (%) | 12 (5.5) | 40 (10.9) |
| Null responder, n (%) | 31 (14.7) | 79 (21.6) |
| Leukocyte × 1,000/mm3 (range) | 6.3 (2.7-13.5) | 6.3 (1.3-12.7) |
| Neutrophil × 1,000/mm3 (range) | 3.3 (0.5-8.6) | 3.1 (0.5-8.2) |
| Hemoglobin g/dL (range) | 14.9 (9.9-18.7) | 15.0 (8.1-18.4) |
| Platelets × 1,000/mm3 (range) | 199 (68-409) | 160 (43-668) |
| < 100,000 platelets, n (%) | 37 (22) | 47 (12.8) |
| AST UI/L | 47.09 ± 29.1 | 80.1 ± 47.6 |
| ALT UI/L | 63.03 ± 50.2 | 103.6 ± 67.3 |
| GGT UI/L | 79.68 ± 85.7 | 113.7 ± 100 |
| Total bilirubin mg/dL (range) | 0.77 (0.16- 2.30) | 0.87 (0.12-3.0) |
| > 2 mg/dL, n(%) | 1 (< 0.5) | 11 (3.1) |
| Serum albumin mg/dL (range) | 4.34 (3.40-5.10) | 4.1 (3.1-5.0) |
| < 3.5 mg/dL, n (%) | 1 (< 0.5%) | 11 (3.1) |
| INR (range) | 1.04 (0.87-1.22) | 0.99 (0.79-1.15) |
BMI: body mass index. There was no patient with albumin below 3.5 g/dL and platelet below 100.000.
Overall, between 22-35% of the cohort was composed of naïve patients while 65-78% of the patients had been previously treated with dual therapy. All patients were distributed based on their previous response profile: 32.5% and 57.4% of relapsers, 5.5% and 10.9% of partial responders, and 14.7% and 21.6% of null-responders had F2 and F3/F4, respectively.
Efficacy resultsThe overall treatment efficacy (n = 582) was 81.6% among patients with F2 and 73.3% among patients with F3/F4 (p = 0.01). Cure rates adjusted to the previous response profile based on the degree of fibrosis were 78.3 vs. 60% among naïve patients, 88.7 vs. 78% among relapsers, 63.5 vs. 65% among partial responders, and 66.7 vs. 41.5% among null-responders for F2 vs. F3/F4, respectively (p = 0.014) (Tables 2 and 4, Figure 1).
The evolution of virologic response during treatment based on the degree of fibrosis and pretreatment type (%).
| Naïve | Relapsers | Partial | Null | p value | |
|---|---|---|---|---|---|
| Undetectable viral load week 4 | |||||
| Mild fibrosis | 35/48 (73) | 96/123 (78) | 3/12 (25) | 21/31 (68) | |
| Advanced fibrosis | 82/129 (63.5) | 73/120 (63) | 20/4 0(50) | 43/79 (54) | 0.003 |
| Undetectable viral load week 12 | |||||
| Mild fibrosis | 41/48(85) | 111/123 (90) | 7/11 (63) | 23/31 (74.2) | |
| Advanced fibrosis | 107/129(83) | 111/120 (92) | 35/40 (88) | 62/79 (78.5) | ns |
| Extended Rapid Virologic | |||||
| Response (eRVR) | |||||
| Mild fibrosis | 31/46(67.4) | 84/116 (72.4) | 3/11* (27.3) | 20/31 (65.5) | |
| Advanced fibrosis | 68/113(60.1) | 65/117 (61) | 18/38 (47.2) | 38/72 (52) | 0.008 |
| Sustained virologic response week 12 | |||||
| Mild fibrosis | 38/48(78.3) | 110/123 (89) | 7/11* (63.6) | 21/31 (67.7) | |
| Advanced fibrosis | 78/129(60.4) | 94/120 (78) | 26/40 (65) | 32/79 (41) | 0.014 |
| Sustained virologic response in patients with eRVR | |||||
| Mild fibrosis | 28/31(90.3) | 82/84 (97.6) | 3/3 (100) | 19/20 (95) | |
| Advanced fibrosis | 63/68(92.6) | 56/65 (86) | 13/18 (72) | 27/38 (71) | 0.003 |
| Sustained virologic response in patients without eRVR | |||||
| Mild fibrosis | 8/16(50) | 21/32 (65.6) | 4/8 (50) | 1/11 (9.1) | |
| Advanced fibrosis | 12/45(27) | 33/42 (63.5) | 8/20 (40) | 5/34 (14.7) | 0.001 |
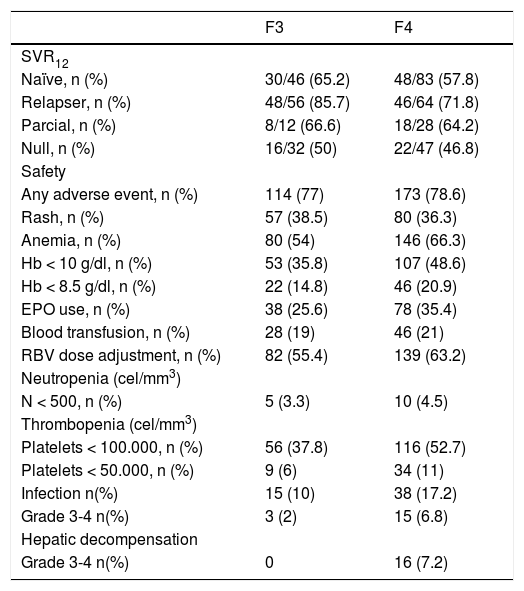
Safety and efficacy for F3 and F4 patients.
| F3 | F4 | |
|---|---|---|
| SVR12 | ||
| Naïve, n (%) | 30/46 (65.2) | 48/83 (57.8) |
| Relapser, n (%) | 48/56 (85.7) | 46/64 (71.8) |
| Parcial, n (%) | 8/12 (66.6) | 18/28 (64.2) |
| Null, n (%) | 16/32 (50) | 22/47 (46.8) |
| Safety | ||
| Any adverse event, n (%) | 114 (77) | 173 (78.6) |
| Rash, n (%) | 57 (38.5) | 80 (36.3) |
| Anemia, n (%) | 80 (54) | 146 (66.3) |
| Hb < 10 g/dl, n (%) | 53 (35.8) | 107 (48.6) |
| Hb < 8.5 g/dl, n (%) | 22 (14.8) | 46 (20.9) |
| EPO use, n (%) | 38 (25.6) | 78 (35.4) |
| Blood transfusion, n (%) | 28 (19) | 46 (21) |
| RBV dose adjustment, n (%) | 82 (55.4) | 139 (63.2) |
| Neutropenia (cel/mm3) | ||
| N < 500, n (%) | 5 (3.3) | 10 (4.5) |
| Thrombopenia (cel/mm3) | ||
| Platelets < 100.000, n (%) | 56 (37.8) | 116 (52.7) |
| Platelets < 50.000, n (%) | 9 (6) | 34 (11) |
| Infection n(%) | 15 (10) | 38 (17.2) |
| Grade 3-4 n(%) | 3 (2) | 15 (6.8) |
| Hepatic decompensation | ||
| Grade 3-4 n(%) | 0 | 16 (7.2) |
Rapid virologic response (RVR) rates, defined as HCV-RNA undetectability at week 4 were higher among patients with F2 (p = 0.002) compared with all other patient groups regardless of the previous response, except for partial responders, with a low number of patients. However, virologic response at week 12 of treatment was similar across patients regardless of the degree of fibrosis. Extended RVR (eRVR: RNA negative at week 4 and week 12) was more frequent among patients with F2 (p = 0.0008), allowing the shortening of treatment in 2/3 of these patients. The vast majority of patients with F2 who showed eRVR achieved SVR12 (Table 2). The multivariate analysis identified the degree of fibrosis (p = 0.002, OR = 1.70, CI95% = 1.20-2.43) and type of response –relapser or naïve– (vs. partial/null responder; p = 0.01, OR = 1.53, CI95% = 1.08-2.43) as the only independent variables associated with eRVR. Extraordinarily high rates of SVR12 (90.3% naïve and 97.6% relapsers) were obtained among patients with F2 who achieved eRVR and were eligible for treatment shortening to 24 weeks (67.4% of naïve patients and 72.4% of relapsers). Only a lower fibrosis stage (F2) (p = 0.014, OR = 1.61, CI95% = 1.06-2.46) and prior relapse (p < 0.0001, OR = 2.43, CI95% = 1.56-3.70) were independently associated with SVR12. No associations were found between SVR12 and age, biochemical or hematological characteristics, viral load, HCV genotype, or IL28B.
Treatment discontinuationIn the whole cohort, treatment discontinuation occurred in 132 patients (Figure 2): 15.8% of patients with F2 and in 26.7% of patients with F3/F4 (p < 0.028). The reasons for discontinuation were categorized into three groups: adverse effects, stopping-rule, and breakthrough. The latter was defined as an increase of 1 log RNA from the previous minimum value recorded or 100UI/ml if RNA was undetectable. The increase treatment discontinuation rate in patients with F3/F4 was related to a higher frequency of viral breakthrough (0.4 vs. 8.1% for F2 vs. F3/F4; p < 0.001) and a slight increase in the number of adverse effects (8.4 vs. 10.8% for patients with F2 vs. patients with F3/F4).
Analyzing viral breakthrough, only one patient with F2 (0.4%) presented breakthrough, compared to 31 patients with F3/F4 (8.3%) who did so. Breakthrough was more common among patients with F3/ F4 (p < 0.0001, OR = 18.51, CI95% = 2.56-142.86) with HCV-RNA > 800,000 IU/l (p = 0.048, OR = 2.32, CI95% = 0.80-6.70) and a detectable viral load at week 4 (p = 0.01, OR = 1.53, CI95% = 1.08-2.43) and patient without eRVR (p = 0.01, OR = 3.70; CI95% = 1.25-11.11). Treatment discontinuation for stopping-rule was similar for both groups (7 vs. 7.5% among patients with F2 vs. those with F3/F4; p = ns).
Adverse effectsTables 3 and 4 summarizes the safety profile of TBT in our study. Significant adverse effects occurred in 67% of patients with F2 and in approximately 78% of patients with F3/F4 (p = 0.01). Serious adverse effects leading to premature treatment discontinuation occurred among 8.4% of patients with F2 vs. 10.8% of those with F3/F4 (p = ns). No patients died during the study. During treatment, 16 patients (4.4%) had hepatic decompensation, all of whom had F3/F4. Other serious adverse effects included grade 3 or 4 rashes in 14 patients, regardless of degree of fibrosis. Infections were reported in 69 patients (11.8%). The development of any type of infection was relatively common during treatment; 16 patients with F3/F4 (5%) had severe infections.
Adverse effects (patients with at least one event).
| Mild fibrosis F2 (n = 214) | Advanced fibrosis F3/F4 (n = 368) | p value | |
|---|---|---|---|
| Any adverse effect, n (%) | 143 (67) | 287 (77.9) | 0.01 |
| Rash: any grade, n (%) | 72 (33.6) | 137 (37.3) | ns |
| Grade 3-4, n (%) | 6 (2.8) | 8 (2.2) | ns |
| Anemia, n (%) | 114 (53.3) | 226 (61.4) | 0.01 |
| Hemoglobin < 10.0 g/dL, n (%) | 58 (27.3) | 160 (43.6) | 0.002 |
| Hemoglobin <8.5g/dL, n (%) | 17 (8.2) | 68 (18.5) | 0.017 |
| Erythropoietin use, n (%) | 54 (25.3) | 116 (31.3) | 0.063 |
| Blood transfusion, n (%) | 25 (11.6) | 74 (20.1) | 0.032 |
| RBV dose adjustment, n (%) | 105 (49) | 221 (60) | 0.01 |
| Neutropenia (cel/mm3) | |||
| N < 750, n (%) | 14 (6.5) | 39 (10.7) | ns |
| N < 500, n (%) | 1 (0.4) | 15 (4.2) | 0.01 |
| Thrombopenia (cel/mm3) | |||
| Platelets < 100,000, n (%) | 64 (30) | 172 (46.7) | 0.0001 |
| Platelets < 50,000, n (%) | 6 (2.7) | 43 (11.7) | 0.0001 |
| Infection, any grade, n (%) | 16 (7.5) | 53 (14.5) | 0.001 |
| Infection grade 3-4, n (%) | 1 (0.4) | 18 (5) | 0.007 |
| Hepatic decompensation (grade 3/4), n (%) | 0 | 16 (4.4) | 0.002 |
| Death, n (%) | 0 | 0 |
The incidences of anemia (Hb < 8.5 g/dL or < 10 g/dL) were significantly higher among patients with F3/F4. More anemia-management interventions occurred for patients with F3/F4; however, the specific timing of the onset of this condition was not recorded.
Similarly, the existence of thrombocytopenia (< 100,000 mm3 or < 50,000 mm3) was more frequent among F3/F4-patients. With respect to neutropenia, differences were only present for neutropenia < 500/ mm3.
DiscussionThe main aim of our study was to compare the efficacy and safety profile of TBT between patients with or without advanced fibrosis (F2 vs. F3/F4) in real life clinical practice. Overall, SVR12 was achieved in 78.4%. SVR rates were significantly higher among patients with F2 compared to F3/F4 (81.6 vs. 73.3%, respectively). Multivariate analysis pointed out that a lower fibrosis stage (F2) and previous relapsers were the only variables independently associated to the achievement of SVR.12 The high efficiency that we obtained in clinical practice is extremely important, especially among patients with F2, which was comparable with that obtained in the registration trials. This efficiency was independent of the unfavorable IL28B and HCV subgenotype. Furthermore, shortening the treatment was possible in 2/3 of naïve patients and relapsers with F2, reaching SVR rates > 90%. TBT can be viewed as a short, and remarkably effective treatment in approximately 70% of naïve patients with F2. Although it is clear that direct comparisons cannot be made between our results and those previously reported, the results of our series (efficiency) were even higher than those observed in registration trials (efficacy).4,16–18
The number of adverse events observed among patients with F3/F4 (67%-287/368) were only slightly higher than those presented by patients with F2 (77.9%-143/214). Regarding severity, patients with F3/F4 (especially those with F4) had significantly more severe adverse events compared to those of F2-patients. Specifically, severe anemia (Hb < 8.5 g/dL) and severe thrombocytopenia (< 50,000 platelets), bacterial infections, and hepatic decompensation were markedly higher among patients with F3/F4.19 In our study, the attending physicians were responsible for anemia management, which was mostly guided by recent publications.20 RBV reduction, the use of erythropoietin, and the number of transfusions were higher among patients with F3/F4 than those with F2. The more aggressive management of this adverse event has most likely yielded better results in practice than in the registration trial protocols with regards to effectiveness and premature treatment termination. Grade 3-4 rash occurred with the same frequency in both groups, which supports an immunoallergic mechanism, independent of the degree of fibrosis. In contrast, the number of severe adverse effects was relatively low among patients with F2, with low impact in terms of treatment discontinuation. This finding was observed despite the fact that our patients were significantly older than those included in the REALIZE21/ ADVANCE trials.4 Clearly, other published studies such as CUPIC22 have higher treatment discontinuation rates because only patients with F4 were included. Infections, particularly respiratory infections, are AEs that have gained special importance. Throughout our study, the interim results of the CUPIC cohort,22 in which patients with albumin < 3.5 g/dL and platelets < 100,000 had a high risk of complications and a poor SVR rate, were known. Although we found patients in our cohort with these characteristics, they were not combined in any patient.
The discontinuation rate found among our patients was relatively low, especially among those with F2. This finding most likely stems from the more aggressive management of adverse effects that is associated with longer treatment durations, resulting in a greater chance of obtaining SVR. In addition, and particularly significant, no breakthrough was observed in patients with F2. This finding clearly influenced the small number of treatment discontinuations among this patient subgroup.
One basic issue that clinicians have faced in recent months and will most likely continue to face in coming years is to decide the immediate treatment or to wait for new generations of DAAs. Multiple variables should be assessed when making this decision including the viral kinetics during the first weeks of treatment, viral load, HCV subtype, IL28B, fibrosis, and the previous response. As we clearly showed in this study, certain combinations of these factors can result in SVR rates > 90% in clinical practice, which are similar to the best results published using IFN-free treatments. In contrast, patients with high viral load, null responders, and those with cirrhosis most likely constitute a subgroup in which triple therapy is no longer useful; however, the SVR figures obtained in our series are relatively high. Undoubtedly, the potential delays in the approval of new drugs by the different regulatory bodies in each country and the possibility of fibrosis progression should also influence this decision. Many previous studies have shown how CHC might progress quickly among patients with F3/F4. Although information is scarce with regard to patients with F2, the relatively rapid progression of disease has been demonstrated in previous cases, particularly when co-infection with HIV exists,23,24 and the risk of underestimating fibrosis is always present. Given its cost-effectiveness and the SVR likelihood of 90% in specific patient subgroups, TBT is an excellent first-line treatment for patients who tolerate IFN and respond to this treatment, even with the advent of new therapies. Furthermore, given that cases of cirrhosis continue to increase in Spain,25 the immediate treatment of patients with F2 will likely reduce the number of patients with cirrhosis.
The positions of the various scientific societies are not uniform with regard to this decision, and it has even changed in recent months. The EASL guidelines published in February 201426 recommend triple therapy with 1st-generation protease inhibitors (telaprevir and boceprevir), whereas the AASLD guidelines recommended treatment with second-generation protease inhibitors (simeprevir) or polymerase inhibitors (sofosbuvir).27 During the April 2014 EASL meeting in London, the concept of rate of change was evident in the new guidelines,28 three months after the previous ones were published. WHO recommendations were also presented during the same conference.11 Furthermore, both recognize the superiority of treatment using new DAAs and the difficulty of their administration in the short-term due to their high cost. In fact, both documents recommend triple therapy with first-generation protease inhibitors. EASL guidelines state, “In settings where none of these options are available, the triple combination of pegylated-IFN-a, ribavirin, and either telaprevir or boceprevir remains acceptable”.28 In this sense, the results observed in our patients with F2 treated with TBT support the difficult balance between the best possible drug and the best currently available drug at a reasonable price, with successful results particularly among naïve patients and relapsers. In the near future, the situation will certainly change, and almost all patients will be treated using IFN-free therapy. At the time of reviewing this paper, simeprevir, sofosbuvir, daclatasvir are becoming available, and also sofosbuvir/ledipasvir and paritaprevir/ritonavir/ombitasvir with dasabuvir. Nevertheless EASL guidelines29 and AEEH guidelines30 include triple therapy among their recommendations.
Therefore, experiences in cohorts of clinical practice are extremely useful for physicians who will begin to use such therapies.
Although our study describes positive results with telaprevir based therapy, certain limitations should be taken into account. First, the current study was based on a sample of patients treated consecutively in different hospitals in Spain, without randomization or external data monitoring. Moreover, the number of F2-patients with partial response is scarce, making it difficult to interpret the results in this subgroup of patients. By the time of reviewing our work, simeprevir, sofosbuvir and daclatasvir are becoming available, so our results could be transferred to these interferon triple combinations, maybe with better results in efficacy and safety.
In conclusion, our results demonstrate that TBT in F2-patients is associated with extraordinarily high SVR rates, treatment duration shortening in more than 2/3 of patients with few and generally mild adverse effects. While all interferon-free regimens are becoming widely available and until the approval of these regimens by regulatory agencies, patients with F2 are most likely the ideal treatment subgroup to receive triple therapy (nowadays simeprevir/sofosbuvir/daclatasvir) because they show the proper balance between treatment risk and benefit.
Abbreviations- •
AE: adverse event.
- •
CHC: chronic hepatitis C.
- •
DAA: direct acting antiviral.
- •
eRVR: early rapid virologic response.
- •
HBV: hepatitis B virus.
- •
HCV: hepatitis C virus.
- •
HDV: hepatitis delta virus.
- •
HIV: human immunodeficiency virus.
- •
RVR: rapid virologic response.
- •
SVR: sustanined virologic response.
- •
TBT: telaprevir-based therapy.
- •
WHO: World Health Organization.
The authors certify that there is no conflict of interests and no financial support.
FundingThis study did not have external funding.