Background/Aim. The pathogenesis and the clinical impact of diastolic dysfunction (DD) in cirrhosis remain unclear. Our aim was to investigate the factors significantly associated with the presence of DD in patients with decompensated cirrhosis on the waiting list for liver transplantation.
Material and methods. consecutive patients with decompensated cirrhosis, who admitted for transplant assessment, were prospectively evaluated. We assessed the independent factors associated with the presence of DD, while their discriminative ability was evaluated by AUC curve. The diagnosis of DD was based on Doppler echocardiography and classified into three categories according to the current guidelines.
Results. we evaluated 115 consecutive patients. Sixty six patients (57.3%-group 1) had DD and 49 (42.7%-group 2) had not DD. The 2 groups had similar Child-Pugh/MELD scores and survival. In multivariable logistic regression analysis, pulse rate (OR: 1.082, 95% CI: 1.03-1.15, p = 0.004), and UNa24h (OR: 0.98, 95% CI: 0.97- 0.99, p = 0.004) were the only variables independently associated with the presence of DD. In the subgroup of consecutive patients (n = 31) with evaluation of cytokines, those (n = 22) with DD, compared to those (n = 9) without DD, had significantly higher levels of inteleukin-6 [145 (45-2000) vs. 56 (10-149)pg/mL, p = 0.043]. Conclusions. We found that DD was independently associated with lower 24-hour urine sodium. Although no correlation was found between DD and severity of liver disease or survival, further studies are needed for final conclusions.
Decompensated cirrhosis (DeCi) is considered a systematic disease affecting the function of several other organs as a result of portal hypertension with splanchnic arterial vasodilation and hyperdynamic circulation. Although alcoholic cardiomyopathy is a well known clinical entity, several studies have shown that cirrhotic patients often develop cardiac dysfunction, which called cirrhotic cardiomyopathy irrespectively of the underlying etiology of liver disease.1 Cirrhotic cardiomyopathy can be characterized by systolic dysfunction (with increased cardiac output but with blunted cardiac reactions to exercise, volume challenge or pharmacological stimuli with vasoconstrictors) and by diastolic dysfunction (DD).1
DD has been associated with increased stiffness of the left ventricular myocardial wall, development of suben- docardial fibrosis and altered cardiac collagen leading to impaired filling during diastole, while the major finding in electrocardiography is the prolongation of the QTc interval.2 DD has been proposed to be associated with fluid overload and sodium retention, severity of ascites and hepatorenal syndrome, while increased levels of cytokines or endotoxins derived from intestinal flora gut have been also implicated in the pathogenesis of DD.3 However, in the literature these variables have never been evaluated in the same cohort of patients with decompensated cirrhosis. Thus, although DD has been observed in a high proportion of cirrhotic patients, its clinical consequences and the exact mechanisms associated with its pathogenesis in patients with decompensated cirrhosis remain unclear.4
The aim of this prospective study in patients with decompensated cirrhosis was to investigate:
- •
Whether DD is related to the severity of liver disease or survival.
- •
The exact factors associated with the presence of DD.
Consecutive adult patients with stable decompensated cirrhosis admitted to our Department for liver transplant assessment between September 2010 and October 2014 were prospectively evaluated. Diagnosis of DeCi was based on the history of ascites, variceal bleeding or encephalopathy in patients with cirrhosis. The presence of ascites was detected by clinical examination and imaging techniques (ultrasound or computer tomography). We excluded patients who underwent liver transplantation (LT) before their admission, but we included those with DeCi and hepatocellular carcinoma (HCC). Patients with a history of active bleeding, encephalopathy or spontaneous bacterial peritonitis (SBP) during the last 1 month before his/her admission were excluded. No patient had known intrinsic cardiac disease and none of them underwent ascitic fluid paracentesis or was receiving β-blockers or furosemide during the last three days before their evaluation.
The following demographic and clinical characteristics were prospectively recorded for each patient on admission: age, sex, cause of liver disease, previous complications of cirrhosis and concomitant disease (e.g. diabetes mellitus, coronary artery disease). On admission to our Department we evaluated the following laboratory variables: hematocrit, white blood count (WBC), platelets (PLT), creatinine, urea, cystatin-C, sodium, potassium, calcium, phosphate, magnesium, protein, albumin, bilirubin, and clotting profile [prothrombin time (PT), INR, activated partial thromboplastin time (aPTT)], ferritin, and lipidemic profile. In addition, the patients were examined in the supine position after an overnight fast in order to evaluate the plasma renin activity (PRA), plasma concentrations of aldosterone and brain natriuretic peptide (BNP). In a subgroup of consecutive patients starting from September 2010, serum levels of interleukin (IL)-1b, IL-6 and tumor necrosis factor (TNF)-a were measured. Finally, calculation of 24-hour urine sodium excretion (UNa24h) was done with collection of 24-hour urine in plastic containers starting at 08:00 am. Samples were centrifuged and sodium concentration was measured, while urine samples were obtained before or after completion of 24-h collection for measurement of random “spot” Na and K concentrations in order to calculate their ratio (UNa/K ratio). Low UNa/K ratio has been associated with the presence of low natriouresis (i.e. 24Una < 78 mmoL/day).5 Severity of liver disease was evaluated by the Child-Pugh (CTP) score6 and MELD score.7
In each patient, assessment of renal function was performed based on serum creatinine using compensated Jaffe (calibrated to be traceable to NIST SRM909b L2) (Beckman Coulter CA 92821, USA) and estimated glomerular filtration rate (eGFR) using the CKD-EPI (chronic kidney disease-epidemiology) creatinine-based equation.8 Measurement of “true” GFR was assessed with 51Chromium-EDTA (51Cr-EDTA) by sampling blood, after intravenous injection of tracer, at 2, 4, and 6 h. “True” GFR was calculated using the slope-intercept technique, correcting for body surface area, and the fast exponential curve recommended by the British Nuclear Medicine Society guidelines.9
Finally, a comprehensive transthoracic echocardiography examination was performed, including tissue Doppler imaging, which is a less heart rate- or load-dependent providing more details about myocardial function. Left ventricular end-diastolic diameter (LVEDD) were recorded as well as the mitral inflow velocity at the tip of the mitral valve leaflet (E- and A-waves, cm/s), while the systolic and early diastolic tissue Doppler imaging velocities in the mitral annulus (sLV, eLV) were assessed. Left ventricular Tei index, which has been used to quantitatively assess myocardial performance,10 was evaluated. The presence of DD was based and classified into three categories [grade I (E/A < 1 and deceleration time (DT) > 240 ms), grade II (E/A < 1 and DT > 140 ms and < 240 ms) and grade III (E/A ratio >1.5 and DT < 140 ms).11 The study protocol was approved by our Institutional Review Board and conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Statistical analysisAll data were analysed using the statistical package SPSS (version 20.0 SPSS Inc, Chicago, IL). The χ2 test was used for comparing qualitative variables and Student i-test and Mann-Whitney U test for comparing quantitative continuous variables. Quantitative variables which were normally distributed were expressed as mean values ± one standard deviation (S.D.) and those non-normally distributed were expressed as median values (range). Multivariable analysis was performed using forward selection of variables, starting with all variables with p < 0.1 in univariate analysis. The discrimination ability of the independent variables to predict the presence of DD in patients with decompensated cirrhosis was evaluated by using the area under a receiver operating characteristic curve (AUC). As the AUC approaches 1.0, the model approaches 100% sensitivity and specificity.12 At the best cut off point (in which the sum of sensitivity plus specificity is maximal), sensitivity, specificity, positive (PPV) and negative (NPV) predictive values were calculated. The patient survival according to presence of DD was calculated using Kaplan-Meier analysis and compared with the log rank sum test. A p value < 0.05 was considered statistically significant.
ResultsIn the present study we included 115 patients with decompensated cirrhosis: twenty four (21%) had HCC, 82 (71%) were male and the mean age was 53 ± 10 years. Viral hepatitis was the cause of cirrhosis in 53 (46%) patients. On admission, the mean CTP and MELD scores were 7.6 ± 1.7 and 13 ± 6, respectively. All patients had a previous history of ascites and all were under diuretics (furosemide: 20-80 mg/day and spironolactone: 50-200 mg/day) until 3 days before their admission. The median values of eGFR-CKD-EPI and “true” GFR were 79 (range: 39-112) and 75 (range: 32-131) mL/min/1.73 m2, respectively.
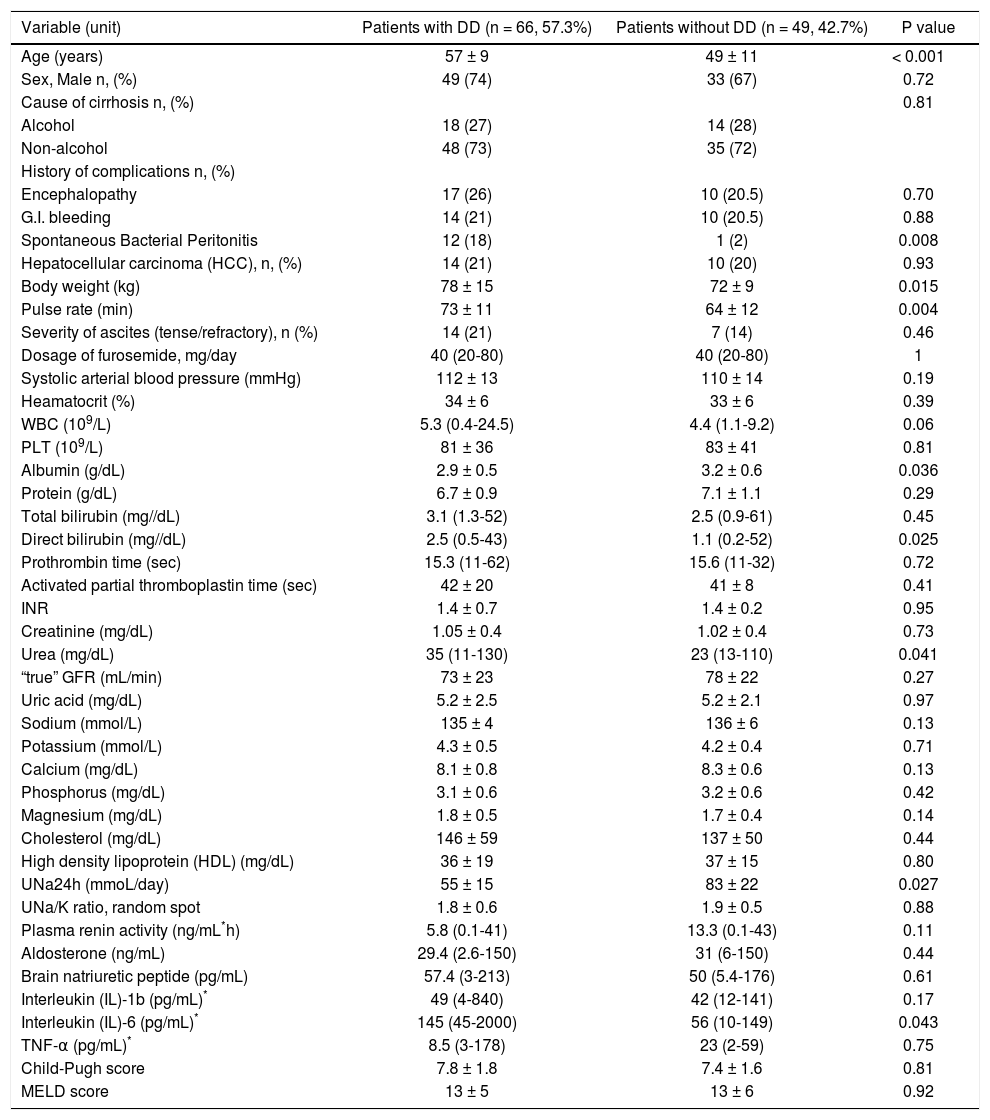
Characteristics of patients with diastolic dysfunctionSixty six patients (57.3%) had DD (40 patients with grade I, 15 patients with grade II and 11 patients with grade III), while 49 (42.7%) had not DD. Patients with DD, compared to those without DD, had more frequently a previous history of spontaneous bacterial peritonitis (SBP) (18% vs. 2%, p = 0.008), were older (57 ± 9 vs. 49 ± 11 years, p < 0.001) and heavier (body weight: 78 ± 15 vs. 72 ± 9 kg, p = 0.015), and they had higher pulse rate (73 ± 11/min vs. 64 ± 12/min, p = 0.004), direct bilirubin [2.5 (0.5-43) vs. 1.1 (0.2-52) mg/dL, p = 0.025] and urea [35 (11-130) vs. 23 (13-110) mg/dL, p = 0.041]. In addition, patients with DD, compared to those without DD, had lower serum albumin (2.9 ± 0.5 vs. 3.2 ± 0.6g/dL, p = 0.036) and 24-hour urine sodium excretion (UNa24h: 55 ± 15 vs. 83 ± 22 mmoL/day, p = 0.027) (Table 1). Interestingly, in the subgroup of consecutive patients (n = 31) with cytokines evaluation, those (n = 22) with DD, compared to those (n = 9) without DD, had similar serum levels of IL-1 and TNF-α, but significantly higher levels of IL-6 [145 (45-2000) vs. 56 (10-149) pg/mL, p = 0.043] (Table 1). However, BNP, plasma renin activity, aldosterone concentration, CTP score and MELD score were statistically similar between the two groups (Table 1). Finally, patients with DD, compared with those without DD (excluding the echocardiographic characteristics in the definition of DD), had significantly higher left ventricular end-diastolic diameter (LVESD: 3.4 ± 0.6 vs. 3.1 ± 0.5 cm, p = 0.028) and Tei index for the left ventricle (0.40 ± 0.1 vs. 0.31 ± 0.1, p = 0.011). All these findings remained unchanged when we excluded the patients with HCC.
- •
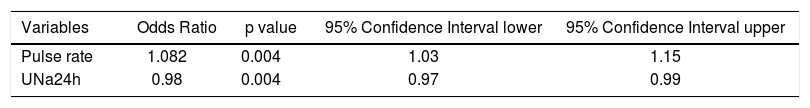
Factors associated with diastolic dysfunction: multivariable logistic regression analysis. In the multivariable analysis, two variables were independently associated with the presence of DD of any grade (grade I-II-III): pulse rate (OR: 1.082, 95% CI: 1.03- 1.15, p = 0.004), and UNa24h (OR: 0.98, 95% CI: 0.97-0.99, p = 0.004) (Table 2). These 2 variables remained significant when HCC patients were excluded from the analysis.
Table 2.Multivariable analysis to identify the independent factors associated with the presence of diastolic dysfunction (DD) in 115 patients with decompensated cirrhosis.
Variables Odds Ratio p value 95% Confidence Interval lower 95% Confidence Interval upper Pulse rate 1.082 0.004 1.03 1.15 UNa24h 0.98 0.004 0.97 0.99 UNa24h: 24-hour urine sodium excretion
- •
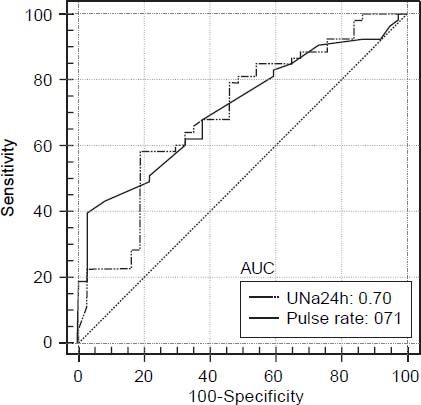
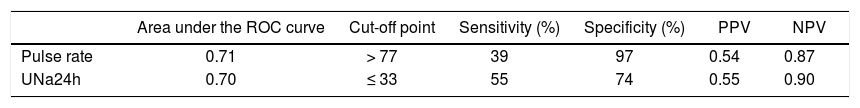
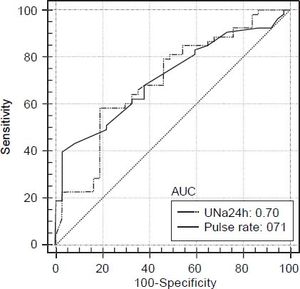
Prognostic factors associated with diastolic dysfunction-receiver-operating characteristics curves. Based on the area under the ROC curves, both UNa24h and pulse rate had good discriminative ability for the presence of any grade DD [AUROC: 0.70 (95% CI: 0.59-0.79) and 0.71 (95% CI: 0.61-0.81), respectively] (Figure 1). The best cut off point for UNa24h was ≤ 33 mmoL/day giving a sensitivity 55%, specificity 74%, PPV 55% and NPV 90%. Finally, the best cut off point for pulse rate was > 77/min giving sensitivity, specificity, PPV and NPV 39%, 97%, 54% and 87%, respectively (Table 3).
Table 3.Prediction of diastolic dysfunction (DD) in 115 consecutive patients with decompensated cirrhosis.
Area under the ROC curve Cut-off point Sensitivity (%) Specificity (%) PPV NPV Pulse rate 0.71 > 77 39 97 0.54 0.87 UNa24h 0.70 ≤ 33 55 74 0.55 0.90 ROC: receiver operating characteristic. PPV: positive predictive value. NPV: negative predictive value. UNa24h: 24-hour urine sodium excretion.
Clinical and laboratory characteristics of patients with decompensated cirrhosis with diastolic dysfunction (DD) vs. those without DD at baseline.
| Variable (unit) | Patients with DD (n = 66, 57.3%) | Patients without DD (n = 49, 42.7%) | P value |
|---|---|---|---|
| Age (years) | 57 ± 9 | 49 ± 11 | < 0.001 |
| Sex, Male n, (%) | 49 (74) | 33 (67) | 0.72 |
| Cause of cirrhosis n, (%) | 0.81 | ||
| Alcohol | 18 (27) | 14 (28) | |
| Non-alcohol | 48 (73) | 35 (72) | |
| History of complications n, (%) | |||
| Encephalopathy | 17 (26) | 10 (20.5) | 0.70 |
| G.I. bleeding | 14 (21) | 10 (20.5) | 0.88 |
| Spontaneous Bacterial Peritonitis | 12 (18) | 1 (2) | 0.008 |
| Hepatocellular carcinoma (HCC), n, (%) | 14 (21) | 10 (20) | 0.93 |
| Body weight (kg) | 78 ± 15 | 72 ± 9 | 0.015 |
| Pulse rate (min) | 73 ± 11 | 64 ± 12 | 0.004 |
| Severity of ascites (tense/refractory), n (%) | 14 (21) | 7 (14) | 0.46 |
| Dosage of furosemide, mg/day | 40 (20-80) | 40 (20-80) | 1 |
| Systolic arterial blood pressure (mmHg) | 112 ± 13 | 110 ± 14 | 0.19 |
| Heamatocrit (%) | 34 ± 6 | 33 ± 6 | 0.39 |
| WBC (109/L) | 5.3 (0.4-24.5) | 4.4 (1.1-9.2) | 0.06 |
| PLT (109/L) | 81 ± 36 | 83 ± 41 | 0.81 |
| Albumin (g/dL) | 2.9 ± 0.5 | 3.2 ± 0.6 | 0.036 |
| Protein (g/dL) | 6.7 ± 0.9 | 7.1 ± 1.1 | 0.29 |
| Total bilirubin (mg//dL) | 3.1 (1.3-52) | 2.5 (0.9-61) | 0.45 |
| Direct bilirubin (mg//dL) | 2.5 (0.5-43) | 1.1 (0.2-52) | 0.025 |
| Prothrombin time (sec) | 15.3 (11-62) | 15.6 (11-32) | 0.72 |
| Activated partial thromboplastin time (sec) | 42 ± 20 | 41 ± 8 | 0.41 |
| INR | 1.4 ± 0.7 | 1.4 ± 0.2 | 0.95 |
| Creatinine (mg/dL) | 1.05 ± 0.4 | 1.02 ± 0.4 | 0.73 |
| Urea (mg/dL) | 35 (11-130) | 23 (13-110) | 0.041 |
| “true” GFR (mL/min) | 73 ± 23 | 78 ± 22 | 0.27 |
| Uric acid (mg/dL) | 5.2 ± 2.5 | 5.2 ± 2.1 | 0.97 |
| Sodium (mmol/L) | 135 ± 4 | 136 ± 6 | 0.13 |
| Potassium (mmol/L) | 4.3 ± 0.5 | 4.2 ± 0.4 | 0.71 |
| Calcium (mg/dL) | 8.1 ± 0.8 | 8.3 ± 0.6 | 0.13 |
| Phosphorus (mg/dL) | 3.1 ± 0.6 | 3.2 ± 0.6 | 0.42 |
| Magnesium (mg/dL) | 1.8 ± 0.5 | 1.7 ± 0.4 | 0.14 |
| Cholesterol (mg/dL) | 146 ± 59 | 137 ± 50 | 0.44 |
| High density lipoprotein (HDL) (mg/dL) | 36 ± 19 | 37 ± 15 | 0.80 |
| UNa24h (mmoL/day) | 55 ± 15 | 83 ± 22 | 0.027 |
| UNa/K ratio, random spot | 1.8 ± 0.6 | 1.9 ± 0.5 | 0.88 |
| Plasma renin activity (ng/mL*h) | 5.8 (0.1-41) | 13.3 (0.1-43) | 0.11 |
| Aldosterone (ng/mL) | 29.4 (2.6-150) | 31 (6-150) | 0.44 |
| Brain natriuretic peptide (pg/mL) | 57.4 (3-213) | 50 (5.4-176) | 0.61 |
| Interleukin (IL)-1b (pg/mL)* | 49 (4-840) | 42 (12-141) | 0.17 |
| Interleukin (IL)-6 (pg/mL)* | 145 (45-2000) | 56 (10-149) | 0.043 |
| TNF-α (pg/mL)* | 8.5 (3-178) | 23 (2-59) | 0.75 |
| Child-Pugh score | 7.8 ± 1.8 | 7.4 ± 1.6 | 0.81 |
| MELD score | 13 ± 5 | 13 ± 6 | 0.92 |
Quantitative variables with normal distribution were expressed as mean values ± one standard deviation and those without normal distribution as median values (range). UNa24h: 24-hour urine sodium excretion;
All patients had a left ventricular ejection fraction > 50%. Patients with grade I DD, compared to those with grade II/III DD, had similar characteristics. However, in the subgroup of patients with cytokines evaluation, it was found that the patients with grade I DD (n = 14), compared to those with grade II/III DD (n = 8), had significantly lower levels of IL-6 [61 (45-292) vs. 280 (71-2000) pg/mL, p = 0.024]. Finally, the patients with grade II, compared to those with grade III DD, had significantly higher random spot UNa/K ratio [1.7 (0.4-4.3) vs. 0.15 (0.1-0.7), p = 0.015]. The latter had excellent discriminative ability for the presence of grade II DD [AUROC: 0.93 (95% CI: 0.79-1.0)], but the number of patients was relatively small for definitive conclusions.
Diastolic dysfunction and mortalityDuring the follow up period [median time 12 (range: 6-52) months], 33 were alive without LT and 82 patients died (n = 39) or underwent LT (n = 43). The causes of death were hepatic failure/multi-organ failure in 28 (68%), followed by HCC (n = 13, 32%). The presence of DD was not associated with worse outcome, since the patients with DD, compared to those without DD, had similar LT-free survival (22/66 or 33% vs. 11/49 or 22.5%, log rank p = 0.83). These findings remained unchanged when only the non-HCC patients were included in the analysis or when the patients with different grade of DD were evaluated.
DiscussionDuring the last years several studies have evaluated the presence of DD in patients with cirrhosis.13 Firstly, it was recognized that DD may become clinically apparent after transjugular intrahepatic portosystemic shunt (TIPS) placement, which can lead to increased cardiac preload and worsening of the left and right end diastolic volumes.14 In these cases, DD has been associated with lower probability of ascites disappearance and survival after TIPS insertion.15 However, the clinical significance of cirrhotic DD in the non-surgical/interventional setting needs further investigation.14 Nevertheless, DD seems to be a relatively common entity affecting more than 50% of the patients with decompensated cirrhosis.16 In our study, we confirmed this finding, since 66 (57.3%) of the 115 patients had DD, but in most cases it was of mild severity (40 patients with grade I and 26 patients with grade II or III).
The factors which are associated with the presence of DD in cirrhotic patients have not been fully elucidated. Advanced age is a known factor correlated to DD17 and in our study we confirmed this finding. However, age was significantly associated with DD only in univariate but not in multivariable analysis. Patients with DD are considered to have a worse hyperdynamic circulation, and thus, higher pulse rate. In our cohort of patients in whom β-blockers had been stopped three days before their evaluation, we found that heart rate was an independent factor for the presence of DD (OR: 1.082, 95% CI: 1.03-1.15, p = 0.004). The discriminative ability of pulse rate was good [AU-ROC: 0.71 (95% CI: 0.61-0.81)] and at the best cut off point > 77 min its specificity and PPV were excellent (97% and 87%, respectively). However, all the other factors related with circulatory dysfunction were not differ- ent in those with versus without DD, including blood pressure, serum BNP, plasma renin activity, aldosterone concentration and renal function assessed with 51Cr-EDTA. In addition, these factors were not different between patients with different grades of DD.
Literature data have indicated that sodium and water retention in cirrhosis play a significant role in the development of DD leading to cardiomyocyte hypertrophy and intramyocardial fibrosis irrespectively of activation of renin-aldosterone system.1,4 Thus, previous studies have shown that a) total removal of the ascitic fluid was led to normalization of cardiac function,18 b) ascitic patients had worse DD, compared to those without ascites19 and c) the presence of severe ascites has been associated with the presence of DD.17 However, similarly to more recent studies,20 in our cohort, we were not able to find a correlation between DD and grade of ascites or severity of renal dysfunction (Table 1), but the patients with DD, compared to those without DD, had significantly lower urinary sodium excretion (UNa24h: 55 ± 15 vs. 83 ± 22 mmoL/day, p = 0.027) and UNa24h was independently associated with the presence of DD (OR: 0.98, 95% CI: 0.97-0.99, p = 0.004). In addition, the patients with grade III, compared to those with grade II DD, had significantly lower spot UNa/K ratio [0.15 (0.1-0.7) vs. 1.7 (0.4-4.3), p = 0.015]. It is known that patients with decompensated cirrhosis have reduced UNa24h and those with refractory ascites characterized by UNa24h less than 78 mmoL/day,21 while UNa/K ratio < 1 has been associated with the presence of UNa24h < 78 mmoL/day.5 In fact, UNa24h and UNa/K ratio may represent more objective indexes of circulatory dysfunction and salt retention than evaluation of severity of ascites in patients with decompensated cirrhosis.5
Recent studies showed that patients with decompensated cirrhosis have high serum levels of cytokines, such as IL-6, IL-1b and TNF-α, as a result of sympathetic activity or bacterial translocation and endotoxemia.14,22 In a recent study by Karagiannakis, ei at.,23 serum levels of lipopolysaccharide-binding protein (LBP), a marker of an exposure to bacterial endotoxin, were associated with the presence of DD. However, no association between LBP and serum levels of cytokines was established.22 In our study, although we evaluated cytokine profile in 31 (27%) of 115 patients due to technical reasons, these patients were consecutive and we found that the patients with DD, compared to those without DD, had significantly higher levels of IL-6 [145 (45-2000) vs. 56 (10-149) pg/mL, p = 0.043]. In addition, the patients with grade II-III DD, compared to those with grade I DD, had significantly higher IL-6 [280 (71-2000) vs. 61 (45-292) pg/mL, p = 0.024]. Although we evaluated cytokine profile in 31 (27%) of 115 patients, they were consecutive patients starting from September 2010.
Similarly to previous studies,17,20,24 we found that the presence of DD was associated neither with the underlying aetiology nor the severity of liver disease, since those with DD, compared to those without DD, had similar CTP (7.8 ± 1.8 vs. 7.4 ± 1.6, p = 0.81) and MELD scores (13 ± 5 vs. 13 ± 6, p = 0.92) (Table 1). However, the former group of patients had more frequently a previous history of SBP (18% vs. 2%, p = 0.008), as well as higher serum direct bilirubin [2.5 (0.5-43) vs. 1.1 (0.2-52) mg/dL, p = 0.025] and lower serum albumin (2.9 ± 0.5 vs. 3.2 ± 0.6 g/dL, p = 0.036) (Table 1). All these variables are well-established indexes of severity of liver dysfunction. However, in the multivariable analysis, none of them were independently associated with the presence of DD (Table 2).
Our study has some limitations including the fact that cytokines were not evaluated in the total cohort and the unavailability of LBP or other serum markers of bacterial translocation/endotoxemia. In addition, in this exploratory study, relatively few patients with grade II or III DD were assessed and most of the patients had low CTP and MELD scores, partly due to the inclusion of patients with HCC.
In conclusion, in this prospective study we evaluated several factors implicating in the pathogenesis of DD in a large cohort of patients with decompensated cirrhosis, and we found that DD was independently associated with lower 24-h urine sodium excretion. Although patients with DD, compared to those without DD, had higher levels of IL-6 and the presence of DD was not associated with the severity of liver disease (evaluated by CTP and MELD scores) and survival, larger studies are needed for final conclusions.
Abbreviations
- •
aPTT: activated partial thromboplastin time.
- •
AUC: receiver operating characteristic curve.
- •
CKD-EPI: chronic kidney disease-epidemiology.
- •
Cr: serum creatinine.
- •
CTP: Child-Turcotte-Pugh.
- •
eGFR: estimated GFR.
- •
GFR: glomerular filtration rate.
- •
HCC: hepatocellular carcinoma.
- •
INR: international normalised ratio.
- •
LT: liver transplantation.
- •
MELD: Model for End-stage Liver Disease.
- •
PLT: platelet count.
- •
PT: prothrombin time.
- •
WBC: white blood count (WBC).
None.
Conflicts of InterestNone.