Background and Aims. The risk of recurrent hepatitis B virus (HBV) infection and prognosis of liver transplantation in patients with HBV has dramatically changed with the use of prophylaxis including hepatitis B immune globulin (HBIg) and antiviral agents.
Methods. This study analyzes the prognostic value of HBV DNA level before orthotopic liver transplantation (OLT) and the effect of HBV prophylaxis on rates of HBV recurrence and survival. Between 1988 and 2008, 859 patients underwent OLT in our center; 60 patients had HBV-related liver disease and in 49, HBV DNA was determined by real time-PCR before OLT. Survival and HBV recurrence were analyzed according to preoperative viral load (HBV DNA <103 lU/mL vs. HBV DNA ≥103) and prophylaxis regimens (HBIg vs HBIg and antivirals).
Results. On multivariate analysis, prophylaxis with HBIg alone, but not HBV-DNA levels was independently associated with poor survival, with a relative risk (RR) of death of 6.5 (95% CI 2.1-19.8, P = 0.001). The risk of HBV recurrence, in this small series, was also associated with monoprophylaxis with HBIg (RR 27, 95% CI 5.2-147.2, P < 0.0001), but not with HBV-DNA levels.
Conclusions. When prophylaxis with HBIg and antiviral agents was administered, survival and HBV recurrence were not influenced by HBV-DNA levels determined by real time-PCR prior to OLT.
Chronic hepatitis B virus (HBV) infection is a common cause of advanced liver disease worldwide and is the indication for approximately 8% of orthotopic liver transplantations (OLTs) performed in Europe and the United States. The long-term outcome and survival following OLT depends on prevention of recurrent HBV infection in the allograft, which is almost universal without HBV pro-phylaxis.1,2
The use of long-term hepatitis B immune globulin (HBIg) prophylaxis plus lamivudine3-11 (LAM) has significantly reduced HBV recurrence after OLT and improved the outcome of transplantation. Currently, the combination of these two agents is the standard prophylaxis used worldwide. However, the dose and duration of HBIg12 are not standardized and vary widely from one transplant center to another.5,13,14
Several factors are associated with a higher risk of recurrent HBV, such as the presence of high HBV DNA levels at the time of transplantation15,16 and drug-resistant HBV.17,23 Other factors, including coinfection with hepatitis delta virus (HDV)24,25 and a diagnosis of fulminant hepatitis are associated with a lower risk.
The assays for measuring HBV DNA levels have improved over the last years. Current polymerase chain reaction (PCR) amplification techniques can detect very low levels of viremia (lower detection limit, 12 IU/mL), whereas the conventional assays used in the majority of studies published to date have a lower limit of 1,000 to 100,000 copies/mL (equivalent to 170-17,000 IU/mL, respectively). In addition, new potent antivirals such as entecavir (ETV) and tenofovir disoproxil fumatare (TDF) have been added to the therapeutic arsenal for patients with chronic hepatitis B.26,27 These factors -better drugs and more sensitive tests to determine HBV DNA- have changed the prophylactic strategies used to prevent HBV recurrence after OLT.28,29
This study analyzes a cohort of patients who underwent OLT for HBV-related hepatitis over a 20- year period in a single center with the aim of evaluating the prognostic role of HBV DNA load at the time of transplantation on the risk of HBV recurrence and the effects of different strategies used for HBV prophylaxis.
Patients And MethodsPatientsAll adult patients who underwent OLT in Hospital Universitario Vall d’Hebron (Barcelona, Spain) from December 1988 to December 2008 were analyzed. Patients were included in the study if they had end-stage liver disease or hepatocellular carcinoma (HCC) associated with HBV infection, defined by the presence of hepatitis B surface antigen (HB-sAg). Following the hospital protocol, patients were submitted to OLT if they had a negative HBV DNA determination by the laboratory assay used at that time. HBV DNA load at the time of OLT was retrospectively analyzed in all available stored serum samples by a quantitative HBV DNA PCR assay.
Medical records were reviewed and data regarding demographics, liver disease, HBV status before and after transplantation, clinical outcome, and survival were collected. Type of HBV prophylaxis and immunosuppression were also analyzed. Patients with concomitant HCC or coinfection with hepatitis C virus (HCV), hepatitis D virus (HDV), or human immunodeficiency virus (HIV) were also included.
Before liver transplantation, all patients were tested for HBV serological markers and HBV DNA. Four preoperative HBV DNA cut-off values were established (<12 IU/mL, < 103 IU/mL, < 104 IU/mL, < 105 IU/mL) to analyze the effect of differing baseline HBV DNA loads on survival.
Following transplantation, routine hematology and biochemistry testing, and HBsAg and antibodies against hepatitis B surface (anti-HBs) were performed every 3 months. When HBsAg was detected in serum, HBV DNA was determined by PCR. HBV recurrence was defined by reappearance of HBsAg in serum in at least 2 separate determinations.
Serological markersHBsAg, IgM anti-HBc antibodies, hepatitis B e antigen (HBeAg), hepatitis B e antibody (anti-HBe), and antibodies against hepatitis C virus (HCV), hepatitis D virus (HDV), and human immunodeficiency virus (HIV) were tested with commercial assays.
HBV DNA determination was not performed routinely in our center until 1991. From that time to 1993, HBV DNA was determined by an inhouse hybridization technique (lower detection limit, 750,000 copies/mL). Between 1993 and 2000, HBV DNA was tested using a qualitative in-house PCR, with a lower detection limit of 103 copies/ mL. Between 2000 and 2007 quantitative HBV DNA testing was done by real-time PCR (Light-Cycler PCR, Roche Diagnostics) with a lower detection limit of 5×102 copies/mL.30 Starting in 2007, HBV DNA has been tested by real-time PCR (COBAS Ampliprep/COBAS TaqMan HBV test assay, Roche Molecular Diagnostics, Basel, Switzerland) with a lower detection limit of 12 IU/mL. Retrospective analysis of HBV DNA in available serum samples frozen (-80 °C) at the time of OLT using this last technique was performed to unify the HBV DNA results.
HBV prophylactic strategiesFrom 1988 to 1996, the standard protocol for prevention of post-OLT HBV reinfection consisted of 10,000 IU of hepatitis B immune globulin (HBIg) intravenously (iv) (Hepatect CP, Biotest Pharma GmbH, Dreieich) during the anhepatic phase and the first day, 5000 IU HBIg iv daily for 5 days, followed by 5000 IU HBIg iv monthly.
From 1997 to 2008, patients received the same HBIg prophylaxis up to day 7, 4000 IU of HBIg intramuscularly (im) weekly (Gamma Anti-Hepatitis B Grifols 1,000 IU, Parets del Vallés, Spain) for 3 additional weeks, and then 2000 IU im monthly indefinitely.31-33 In this second period, all patients were additionally treated with an oral nucleos(t)ide analogue (NUC), initiated before or after OLT and continued indefinitely.
Immunosuppression protocolThe immunosuppression therapy differed according to the year of transplantation. From 1988 to 1996, the immunosuppressive regimen was mainly based on cyclosporine (CyA). Tacrolimus (Fk) was introduced and combined with CyA between 1997 and 1998, and since 1999, Fk has been used exclusively. Steroids were given until 2004, and from that time on, mycophenolate mofetil (MMF) has been used. Steroids were gradually reduced and discontinued within 3 to 6 months following OLT.
Statistical analysisData are presented as the mean ± standard deviation or median (interquartile ratio), according to the distribution of the variables. Normality was determined by the Shapiro-Wilk test. Associations between categorical variables were examined with the chi-square or Fisher exact test. Parametric or non-parametric tests (Mann-Whitney U test) were used to determine the associations between quantitative variables. A Cox proportional hazards regression model was applied to identify variables associated with recurrence or survival during the follow-up period. Forward stepwise Cox regression was used to identify variables independently associated with recurrence or survival according to the likelihood ratio (inclusion criteria for the model = 0.05, tolerance = 0.1). Data were analyzed with SPSS software (15.0, SPSS Inc., Chicago IL, USA).
ResultsOver the 20-year study period, 859 patients underwent OLT, and 60 cases (7%) were related to HBV infection. The indications for OLT were endstage liver disease in 29 (59%) and HCC in 20 (41%) patients. There were no cases of fulminant hepatitis B infection. Eleven patients were excluded from the study. One patient died during surgery, and in 10 patients, HBV DNA determination by a sensitive PCR assay was not performed before OLT. Hence, 49 patients followed up for a median of 45 months post-OLT (2-152 months) were included in the analysis. Baseline characteristics of the patients are shown in Table 1. Preoperative HBV DNA levels are shown in Table 2.
Pretransplantation patient characteristics.
| No. patients | 49 |
|---|---|
| Sex, males | 43(88%) |
| Mean age ± SD, y | 52 ± 9.8 |
| Mean ALT ± SD, IU/mL | 76 ± 51 |
| HBeAg-positive | 9(18°%) |
| HBV DNA undetectable, < 12 IU/mL | (18°%) |
| HBV DNA ≤ 103 IU/mL | 39(80%) |
| Median HBV DNA (range), log IU/mL | 102(0.108) |
| HCV RNA-positive | 5(10%) |
| HDV RNA-positive | 5(10%) |
| Anti-HIV-positive | 1 (2%) |
| Hepatocellular carcinoma | 20(41%) |
| Child-Pugh A | 11 (22%) |
| Child-Pugh B | 19(39%) |
| Child-Pugh C | 19(39%) |
| Pre-OLT oral antivirals | 29(59%) |
| Median duration of preoperarative | |
| oral antiviral therapy (range), days | 362(24-1292) |
Pre-liver transplant HBV-DNA levels and HBV recurrence.
| Recurrence | P | |
|---|---|---|
| HBV-DNA < 12 IU/mL | 1/18(6%) | 0.22 |
| HBV-DNA ≥ 12 IU/mL | 7/31(23%) | |
| HBV-DNA < 103 IU/mL | 3/39(8%) | 0.005 |
| HBV-DNA ≥ 103 IU/mL | 5/10(50%) | |
| HBV-DNA < 104 IU/mL | 54/42(12%) | 0.07 |
| HBV-DNA ≥ 104 IU/mL | 3/7(43%) | |
| HBV-DNA < 103 IU/mL | 7/46(15%) | 0.42 |
| HBV-DNA ≥ 103 IU/mL | 1/3(33%) |
The 49 patients received 2 different prophylaxis regimens: 7 (14%) received HBIg alone, all in the period of 1988 to 1996, and 42 (82%) received the combination of HBIg and an oral antiviral, all in the period of 1997 to 2008. The antiviral drug administered was LAM in 38 patients, ETV in 2, TDF and emtricitabine (FTC) in 1, and the combination of LAM and adefovir (ADF) in 1 patient.
Post-OLT immunosuppression was based on CyA and steroids in 10 patients (20%), and Fk either with steroids or MMF in 39 patients (80%). Acute cellular rejection occurred in 13 patients (26%) and was treated with 500 mg methylprednisolone for 3 days.
Survival resultsThirteen patients (26%) died after OLT. In 4 patients (30%), all of whom were receiving HIBg alone, death was related to HBV recurrence. In the remaining 9 patients, the cause of death was HCC recurrence (N = 3), primary non-function (N = 1), gastric adenocarcinoma (N = 1), graft versus host disease (N = 1), and biliary sepsis (N = 3) (Figure 1).
The variables associated with poor survival in the univariate analysis were HBV DNA levels ≥103 IU/ mL prior to OLT (P = 0.01), HBIg monoprophylaxis (P = 0.001), and HBV recurrence post-OLT (P = 0.006). Coinfection with HCV, HDV, or HIV was not associated with survival. In the multivariate analysis, the only variable related with a poor outcome was HBIg monoprophylaxis, which was associated with an RR of death of 6.5 (95% CI 2.1-19.8, P = 0.001).
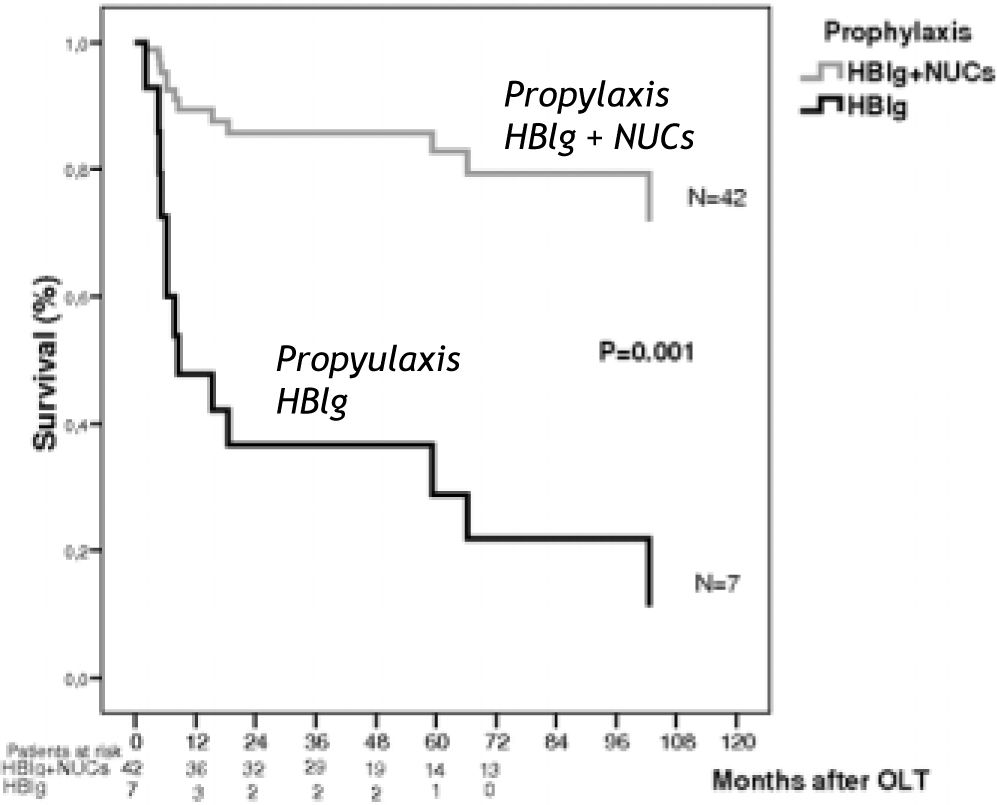
Overall 3-year and 5-year survival rates for the group receiving HBIg were 36% and 20%, respectively. Survival rates in the group receiving HBIg plus oral antiviral prophylaxis were 87 and 82%, respectively (P = 0.001) (Figure 2).
HBV recurrenceEight of the 49 patients studied (16%) had recurrent HBV infection, including 5 (71%) receiving HBIg and 3 (7%) receiving HBIg and antiviral prophylaxis (Table 1).
Descriptive data and outcome of patients with HBV recurrence. *Patient with poor treatment adherence.
| Patient | Pre-OLT HBV DNA(IU/mL) | Prophylaxys type | Time to recurrence(days) | Outcome |
|---|---|---|---|---|
| 1 | 2.82 × 103 | HBIg | 152 | Died-Cholestatic hepatitis |
| 2 | 4.3 × 104 | HBIg | 63 | Died-Cholestatic hepatitis |
| 3 | 1.58 × 104 | HBIg | 196 | Died-Cholestatic hepatitis |
| 4 | 4.14 × 104 | HBIg | 13 | Died-Diffuse HCC |
| 5 | 1.29 × 105 | HBIg | 277 | Alive-LAM + ADF treatment |
| 6 | 1 × 103 | HBIg + LAM | 657 | Alive-LAM + ADF treatment |
| 7 | 3.2 × 102 | HBIg + LAM | 211 | Died-HCC recurrence |
| 8 | < 12 | HBIg + ETV | 148 | Died-HCC recurrence |
None of the following variables were associated with a higher risk of HBV recurrence: age, sex, Child-Pugh score, concomitant HCC, HBeAg-positive status, HCV or HDV coinfection, rejection, or pre-OLT antiviral treatment.
Patients with HBV DNA ≥104 IU/mL had a higher risk of HBV recurrence, without reaching statistical significance (P = 0.07).
Variables associated with recurrence in the univariate analysis were preoperative HBV DNA levels ≥ 103 IU/mL (P = 0.004), HBIg monoprophylaxis (P < 0.0001), and immunosuppression with CyA (P = 0.03). The risk of HBV recurrence according to preoperative HBV DNA levels is shown in Figure 3. There were no differences in HBV recurrence according to preoperative HBV DNA levels in patients receiving HBIg plus oral antiviral agents (P = 0.32).
In the multivariate analysis, the only variable significantly associated with a higher risk of HBV recurrence was the type of prophylaxis. HBIg monoprophylaxis was associated with an RR of recurrent HBV of 27 (95% CI 5.2-147.2, P < 0.0001).
The median time to recurrence was 47.01 (IR 87.7-29.2) months in the group receiving HBIg and oral antivirals and 4.9 (IR 9.1-2) months in the group receiving HBIg alone (P < 0.0001).
HBV recurrence at 3 and 5 years was 5% for patients receiving HBIg and oral antivirals. In the group receiving HBIg there was a high rate of recurrent HBV and death; hence patients were at risk for 15 months. At that time, recurrence was 83% (P < 0.0001) (Figure 4). Of note, in both groups all events except one (in the patient with poor adherence to treatment) occurred during the first year posttransplantation.
The single long-term survivor in the group receiving prophylaxis with HBIg experienced HBV recurrence 277 days after OLT. Treatment with LAM was initiated and ADF was later added because of the emergence of HBV resistant strains to LAM. The patient’s liver function improved and HBsAg tested negative 7 years after recurrence.
In the group that received prophylaxis with HBIg and oral antivirals, 3 patients had HBV recurrence; preoperative HBV-DNA load was <103 IU/mL in 2 cases, and < 12 IU/mL in 1 case. HBV recurrence was associated with poor adherence to LAM treatment in 1 patient who had an episode of acute hepatitis B infection 657 days after OLT that was treated with LAM and ADF. HBV-DNA became undetectable and persisted negative after 5 years of follow up. The second patient, also receiving HBIg and LAM, experienced asymptomatic HBV recurrence 211 days after OLT. At that time ALT levels were normal and HBV-DNA was undetectable. The patient was treated with LAM, but he presented HCC recurrence and died 261 days following OLT. The third patient was under treatment with HBIg and ETV. HBV recurred 138 days after transplantation, with normal ALT levels and undetectable HBV-DNA. He continued with ETV, but presented HCC recurrence and died 261 days after OLT.
Three patients had high HBV-DNA levels at the time of transplantation (≥ 105 IU/mL); 2 received HBIg prophylaxis and 1 had HBV recurrence. One patient receiving HBIg and LAM prophylaxis did not present recurrence.
DiscussionThis study analyzes the risk of HBV recurrence following OLT in 49 patients who underwent transplantation in a single Spanish center. The rate of recurrent HBV in the group of patients who received HBIg and an oral antiviral drug was 7%, a value clearly lower than the overall risk of 16%, indicating that this combination prevents HBV recurrence in most cases, even in patients with detectable viremia at the time of transplantation. In our study, the risk of HBV recurrence was strongly influence by the type of prophylaxis administered and the year in which liver transplantation was performed. These variables are strongly interrelated. During the first years of the transplant program, HBIg was administered in a less strictly way than in the following years. Moreover, HBV DNA determination was initially performed by hybridization tests followed by in-house PCR techniques, which were less sensitive that the currently used real-time PCR techniques. These factors may explain the high recurrence rate (71%) observed between 1988 and 1996. Implementation of combined HBV prophylaxis has completely changed the scenario, reducing the HBV recurrence rate to less than 10%.
For years there has been controversy around the issue of whether patients with HBV DNA levels higher than 100,000 copies/mL should be candidates for liver transplantation, particularly in the era of HBIg monotherapy, when the risk of HBV recurrence was clearly related to the viral load. In 2005, Marzano, et al.15 reported a linear correlation between HBV recurrence and viral load at the time of surgery. The authors analyzed viral load by a single amplification assay with a DNA detection limit of 200 copies/mL and found that the risk of HBV recurrence was 50% in transplant patients with HBV DNA higher than 100,000 copies/mL, 7.5% in those with 200 to 99,999 copies/mL, and 0% in patients with undetectable HBV DNA. These results suggested that a pretransplantation viral load higher the 100,000 copies/ mL was significantly associated with hepatitis B recurrence.
Our study has the advantage that HBV DNA levels were measured with a very sensitive commercial PCR technique with a wide linearity range which enables detection of low and high viral levels, yielding results that are similar to those observed in current clinical practice, and shows that HBV DNA levels does not influence survival and HBV recurrence when prophylaxis with HBIg and NUCs was administered.
However, the study has several limitations. It is a retrospective analysis with data spanning a lengthy period, HBV prophylaxis has changed, the sample of patients included was heterogeneous, and few patients received potent antivirals, such as ETV and TDF. However, it has the advantage of being performed in a single center, the same technique was used for HBV DNA determination, and the clinical practice and follow-up were similar.
Overall, the HBV recurrence rate was high (16.3%), but if the results are broken down by time periods, there was a dramatic decrease in HBV recurrence rate from the first period (1988-1996; 71%), to the second period (1997-2008; 7%). A factor that strongly influenced HBV recurrence was the type of prophylaxis administered in each period. Twenty years ago, the introduction of HBIg began a new era that dramatically changed the evolution of HBV patients after liver transplantation.1,2 Another advance was achieved with the introduction of oral antiviral prophylaxis,3,11 which yielded even better results, enabling preoperative inhibition of viral replication and reducing recurrence rates. The greatest experience in this setting is with LAM. The main drawback of this drug is the fact that if a prolonged pretransplantation regimen is needed, there is a risk of emergence of drug-resistant HBV strains that can compromise liver transplantation and facilitate HBV recurrence.17,23,29 Currently, more potent drugs with a high genetic barrier such as ETV and TDF are expected to change the management of these patients.
In the present study, OLT outcome in patients with preoperative viral replication receiving HBIg and LAM did not significantly differ from that of patients with undetectable HBV DNA. Although the preoperative HBV DNA level was statistically significant in the univariate analysis, we believe that it lost strength when the potent prophylaxis strategy was applied.
The univariate analysis showed and association between HBV DNA levels ≥103 IU/mL and a higher risk of HBV recurrence (P = 0.004), and only a trend in patients with HBV DNA ≥ 104 IU/mL (P = 0.07). This could be related to a type 2 error due to the relatively small number of patients in our study.
The only patient in our cohort with an HBV-DNA level ≥ 105 IU/mL who received HBIg and LAM did not experience HBV recurrence. This contrasts with results from earlier reports1,34,35 but is similar those described in recent studies.24,28,29,36 Hence, the presence of viral replication should not be considered an absolute contraindication for liver transplantation if an adequate postoperative prophylaxis is used.28,29 In effect, by testing stored samples with current, more sensitive assays, we now know that they had detectable viral replication.
One interesting observation was the higher risk of developing recurrent HCC in patients with recurrent HBV, as has been reported previously.37,38 Two patients with HBV recurrence in our series died due to HCC recurrence. Both were only HBsAg-positive, HBV-DNA was undetectable, and they were receiving HBIg and oral antiviral agents. The outcome in these patients suggests that HBIg and oral antivirals are only partially effective in preventing HBV reinfection in patients with recurrent HCC.38 A recent study39 has shown that covalently closed circular DNA was the main type of HBV-DNA isolated from tissues of patients with HCC, and its presence in HCC cells and in nontumor cells, suggesting that replication can occur in tumor cells. Identification of HBV-DNA in extrahepatic sites in the absence of HBV DNA in liver and serum indicates that viral replication may occur in occult sites, thereby enabling HBV recurrence.
In conclusion, in patients with chronic hepatitis B infection, OLT has an excellent 5-year survival rate (82%) and low 5-year risk (5%) of HBV recurrence when combination prophylaxis with HBIg and an antiviral agent is administered, regardless of pretransplantation HBV DNA levels.
AcknowledgmentsWe thank Celine Cavallo for English language revision and Esther Delgado for her secretarial work.
The authors declare that they have received no funding for this study and have no conflicts of interest.
Abbreviations- •
HBV: Hepatitis B virus.
- •
HCV: Hepatitis C virus.
- •
HDV: Hepatitis D virus.
- •
HBIg: Hepatitis B immune globulin.
- •
OLT: Orthotopic liver transplantation.
- •
HBsAg: Hepatitis B surface antigen.
- •
HBeAg: Hepatitis B e antigen.
- •
anti-HBe: Hepatitis B e antibody.
- •
HIV: Human immunodeficiency virus.
- •
HCC: Hepatocellular carcinoma.
- •
LAM: Lamivudine.
- •
ADF: Adefovir dipivoxil.
- •
ETV: Entecavir.
- •
TDF: Tenofovir disoproxil fumatare.
- •
FTC: Emtricitabine.
- •
PCR: Polymerase chain reaction.
- •
CyA: Cyclosporine.
- •
Fk: Tacrolimus.
- •
MMF: Mycophenolate mofetil.
- •
NUCs: Nucleos(t)ide analogues.