Background and rationale. Acute and chronic heart failure (HF) may affect the liver, but the underlying mechanisms that lead to progressive liver damage are poorly understood. The hepatic cytokeratin-18 (CK18) epitopes M65 and M30 have been reported to distinguish between overall (necrotic) and apoptotic cell death, respectively. We aimed to evaluate the predominant hepatic cell death pattern in acute vs. chronic heart failure and examined if these assays predict the course of the disease.
Main results. In a prospective study comprising 21 patients with acute HF (AHF) and 18 patients with chronic HF (CHF) serum levels of M65 and M30 were assessed. Compared with CHF, M65 levels were significantly increased in patients with AHF (CHF: 1,283 ± 591.6U/l vs. AHF: 20,912 ± 15,132U/l, p > 0.001). In addition, M30 levels were significantly increased in AHF (CHF: 642.2 ± 177.4U/l vs. AHF: 3,844 ± 5,293U/l, p < 0.05), but the M30/M65 ratio was significantly higher in CHF (CHF: 0.54 ± 0.15 vs. AHF: 0.20 ± 0.19, p < 0.001), indicating a greater contribution of apoptotic cell death in CHF. AHF patients with higher M30 values had a worse prognosis.
Conclusions. The ratio of CK18 M30/M65 is a potential marker to discriminate AHF from CHF induced LF and M30 might be a prognostic marker for survival in AHF induced liver injury.
Acute liver failure (ALF) is defined as an abrupt onset of jaundice and coagulopathy in the absence of pre-existing liver disease. Various etiologies (toxic, viral, secondary, etc.) have been identified to induce ALF, including heart failure (HF).1 Liver abnormalities are common in heart disease and typically seen in patients with a cardiac index of below 1.5 l/min/ m2.2 Chronic heart disease causes liver injury that may further progress to cardiac cirrhosis and cardiogenic ischemic hepatitis.3 Interestingly, although hepatic symptoms in this patient cohort are generally mild and right-ventricular heart failure related symptoms predominate, serum liver function tests (LFT’s) are of good prognostic value.4
In contrast, acute cardiac congestion secondary to myocardial infarction or major surgical interventions frequently causes a dramatic increase in LFT’s.5 Such liver injury often poses a diagnostic and therapeutic dilemma, since it might progress to ALF and it is generally difficult to distinguish from other causes of liver injury. In patients without obvious symptoms of heart failure, the exclusion of other known causes of ALF might consume valuable time necessary for clinicians to restore cardiac function.
Since hepatic congestion and ischemia initiate a cascade of events that ultimately result in hemorrhagic centrilobular destruction of liver tissue with hepatocyte death, we aimed to study the exact patterns of hepatocyte damage in patients with acute vs. chronic heart failure. We recently reported that the pattern of hepatocyte cell death (i.e. necrosis vs. apoptosis) was of prognostic value in ALF. Furthermore, cell death markers were significantly elevated in patients with HF-associated liver injury compared with healthy individuals and helped distinguish cardiogenic from other (i.e. HBV) causes in this cohort.6 Here, we report that peripheral cell death markers help to discriminate between acute and chronic heart failure induced liver injury and propose that M30 might be a prognostic marker for survival in patients with heart failure.
Experimental ProceduresPatientsThe study was carried out according to the Declaration of Helsinki and the guidelines of the International Conference for Harmonization for Good Clinical Practice. We included 21 consecutive patients with ALF due to acute HF (AHF), which was diagnosed by right heart catheterisation, transthoracic echocardiography and laboratory data. We furthermore included 18 consecutive patients who were referred to the II. Department of Medicine, University Hospital Mainz, with the established clinical diagnosis of chronic HF (CHF) and consecutive liver injury (LI). Since heart failure induced LI is a subacute secondary condition, we applied the term liver injury to the patients rather than ALF in the acute setting. All patients had presented without apparent pre-existing liver disease and were otherwise excluded.
Laboratory dataSera from CHF patients were collected during routine check-up presentation in the outpatient clinic. Sera from AHF patients were collected upon admission and throughout hospitalization. All sera were stored within 2h at -20 °C until testing. Individual values of clinical and standard laboratory data, markers of overall cell death and apoptosis (M65 ELISA and M30-Apoptosense, respectively, both Peviva; Bromma, Sweden) were determined.
Standard procedure for patients with acute liver injuryPatients, who were admitted with the diagnosis of LI underwent the following diagnostic procedures: an ultrasound Doppler, in particular of the liver, to exclude an acute Budd-Chiari syndrome, blood flow measurements were performed using sonographic equipment with colour Doppler capability (Siemens Sonoline, Erlangen, Germany) and a 3.5MHz probe to determine tracing in the portal vein, the hepatic artery and the hepatic vein. Moreover, special laboratory investigation for all other possible diagnoses that are associated with ALF were performed, e.g. viral hepatitis (A and B and E), autoimmune hepatitis, M. Wilson, drug-related toxicity (in particular acetaminophen). Laboratory routine workup was performed daily and included at least total bilirubin, AST, ALT and INR.
StatisticsDifferences between laboratory values were evaluated by one-way ANOVA, repeated-measure ANOVA, or paired Student’s t-test and t-test for independent-samples. For categorical variables, frequencies and percentages were estimated. ϰ2 or Fisher’s exact tests were used for categorical factors. ROC calculations were undertaken where applicable. Screening, optimal and diagnostic cutoff values were calculated. All values are given as means ± standard errors of means. Analyses were performed with SPSS 17.0.1, version 2008 (SPSS, Chicago, IL, USA).
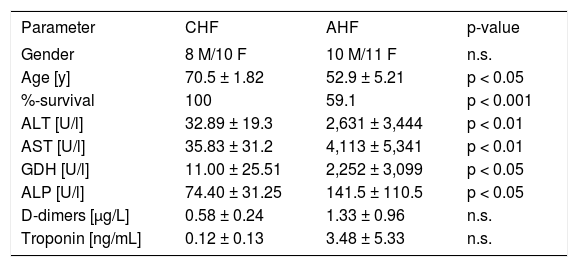
ResultsPatients and clinical outcomeWe included 21 cases of acute LI (10 males, 11 females) aged 52.9 ± 5.21. The diagnosis of AHF and/ or cardiogenic shock at admission was associated with a mortality rate of 40.9 %. Leading cause of death was the underlying heart failure. We found significantly elevated serum concentrations of ALT, AST, ALP and GDH in the AHF-patients compared to CHF patients (Table 1). The serum markers troponin and D-dimers were also increased in AHF, but this difference did not reach significance. CHF patients were older and had a lower mortality rate.
Demographic and basic clinical data of CHF and AHF patients.
| Parameter | CHF | AHF | p-value |
|---|---|---|---|
| Gender | 8 M/10 F | 10 M/11 F | n.s. |
| Age [y] | 70.5 ± 1.82 | 52.9 ± 5.21 | p < 0.05 |
| %-survival | 100 | 59.1 | p < 0.001 |
| ALT [U/l] | 32.89 ± 19.3 | 2,631 ± 3,444 | p < 0.01 |
| AST [U/l] | 35.83 ± 31.2 | 4,113 ± 5,341 | p < 0.01 |
| GDH [U/l] | 11.00 ± 25.51 | 2,252 ± 3,099 | p < 0.05 |
| ALP [U/l] | 74.40 ± 31.25 | 141.5 ± 110.5 | p < 0.05 |
| D-dimers [μg/L] | 0.58 ± 0.24 | 1.33 ± 0.96 | n.s. |
| Troponin [ng/mL] | 0.12 ± 0.13 | 3.48 ± 5.33 | n.s. |
To elucidate if cell death markers were associated with classic liver parameters, correlation analysis was performed. In CHF, M65 significantly correlated with serum AST (Figure 1A) and ALP (Figure 1B); the latter was also correlated with serum M30 (Figure 1C). No correlation was observed between cell death markers and liver parameters for AHF patients. Intriguingly, the surrogate marker for myocardial damage, myoglobin, significantly correlated with serum M30 in AHF (Figure 1D).
Correlation of classic liver function parameters with cell death markers. In chronic heart failure (CHF) M65 correlated significantly with AST (A) and ALP (B). In addition M30 was correlated with ALP (C). In acute heart failure (AHF) myoglobin was significantly correlated with M30 (D).
As previously published, cell are death markers were elevated in both CHF- and AHF-patients, indicating that a large number of cells are undergoing apoptotic and necrotic cell death.6 Levels of M65 and M30, however, were significantly higher in AHF (M65: chronic 1,283 ± 591.6U/l vs. acute 20,912 ± 15,132U/l, p < 0.001; M30: chronic 642.2 ± 177.4U/l vs. acute 3,844 ± 5,293U/l, p < 0.05; Figure 2A and B).
Apoptosis is the predominant cell death mechanism in CHF-induced liver injuryPatients with CHF exhibited a significantly higher M30/M65-ratio than AHF patients (M30/ M65: chronic 0.54 ± 0.15 vs. acute 0.20 ± 0.19, p < 0.001; Figure 2C), suggesting that a greater proportion of cells are undergoing apoptotic cell death in CHF. In contrast, AHF-patients were polarized towards necrotic cell death.
Cell death markers may predict survival in AHFIn order to evaluate the potential of the analysed markers to predict the outcome after AHF, we grouped the patients into survivors (n = 9) and non-survivors (n = 13). We found a trend for increased M30-levels (survivors: 1,115 ± 408 non-survivors: 5,734 ± 2,106; p = 0.06; Figure 3A) and the M30/M65 ratio (survivors: 0.08 ± 0.04, non-survivors: 0.25 ± 0.07; p = 0.08; Figure 3B) in non-survivors vs. survivors. D-dimers, which have previously been shown to correlate with survival in HF differed significantly between the groups (survivors: 0.69 ± 0.20, non-survivors: 2.38 ± 0.22; p < 0.001; Figure 3B).7
Potential prognostic value of cell death for outcome after acute congestive heart failure. Patients were grouped as survivors (n = 9) or non-survivors (n = 13). While no significant difference for M30 (A) as well as the M30/M65 ratio (B) were observed, d-dimers differed significantly between the groups (B).
In this study, we analysed patients with LI secondary to either AHF or CHF and found substantial differences in cell death mechanisms. HF induces hepatocyte apoptosis and/or necrosis and thus triggers impaired liver function, which is a hallmark of acute LI. Patients with LI, secondary to AHF had significantly higher serum CK18 levels (cleaved and uncleaved) compared to patients with CHF. However, the M30/M65 ratio, representing the ratio between apoptosis and necrosis was higher in CHF patients. This is of clinical relevance and helps distinguish the cause of liver injury in cardiologic patients. Furthermore, we found that M30 ELISA might be utilised to distinguish patients with good vs. bad prognosis. However, a larger cohort of patients would be required to validate these results.
Recent studies reported an increase in caspase cleaved CK18 in the serum of patients admitted for acute myocardial infarction (AMI).8 The M30 assay was utilised in a study of 39 patients with AMI, and was shown to be superior to troponin T and creatine kinase in the diagnosis of AMI.9 An abundance of CK18 antibodies is seen in patients with coronary heart disease.10 However, the origin of the CK18 remains unclear; in one study, weak staining for CK18 in cardiomyocytes virtually excluded the cardiomyocyte as the source of caspase cleaved CK18, despite the identification of cleaved CK18 fragments within myocardial lysosomes.11 The authors concluded that the source of the cleaved CK18 was not the myocardium, but rather endo-thelial cells or extracardial tissues. Indeed, macrophage uptake of serum cleaved CK18 was suggested to account for the abundance of M30 positive material within the myocardium. No studies, however, examined for the extra-cardiac, or hepatic contribution to CK18 levels. Since we and others have shown that liver injury is associated with the systemic release of cleaved and uncleaved CK18, and that M30 levels correlate with serum LFT's rather than markers for myocardial damage, this study suggests the hepatocyte is the primary source of CK18 epitopes in AMI.1213
While systemic hypotension or shock may induce LI in a patient with cardiac dysfunction, additional mechanisms contribute to LI. For example, HF resulting in increased central venous pressure and hepatic venous congestion induce sinusoidal dilation, endothelial injury, replacement of hepatocytes with erythrocytes, and ultimately centrilo-bular tissue destruction.5 Consistent with this, Lebray et al. observed increased liver stiffness, unrelated to hepatic fibrosis in a patient with HCV who underwent heart transplant.14 Millonig et al. further reported that the clamping of the inferior vena cava in pigs led to a significant increase in liver stiffness, while treatment of congestive cardiac failure in patients led to the amelioration of liver stiffness, as assessed by transient elastography.15 Interestingly, changes in liver stiffness did not correlate with serum LFT’s. In our cohort of CHF patients, serum LFT’s were also within normal limits, but M30/M65 ratio indicated an apoptotic cell death pattern. Thus, these assays might be a more sensitive screening tool for hepatocyte damage in hepatovenous congestion compared to standard LFT’s. Although it may be difficult to ascertain the source of CK18, the pattern of cell death markers could be a useful predictive marker of hepatic outcomes.16
In contrast, patients with AHF exhibit high serum levels of cell death markers and highly elevated LFT’s. This common clinical constellation is clearly dominated by the primary cardiac event and cardiologic/ cardiothoracic surgical treatment is the curative management of choice. However, we identified the serum M30-epitope and the ratio of M30/M65 as possible prognostic markers for the survival of AHF patients. These results are consistent with previous studies that demonstrated serum LFTs to be of good prognostic value in heart disease.4 Further validation of serum cell-death markers in AHF and CHF patients will be important to help identify HF-patients at risk of severe liver injury, particularly in the pre-operative setting.
Financial SupportThis work was supported by the Deutsche Forschungsgemeinschaft (DFG, grant 267/8-1), the Wilhelm Laupitz Foundation and the IFORES program of the University of Essen (L.P.B.)
Conflict of InterestNone declared.
Abbreviations- •
ALF: Acute liver failure.
- •
LI: Liver injury.
- •
HF: Heart failure.
- •
AHF: Acute heart failure.
- •
CHF: Chronic heart failure.
- •
CK18: Cytokeratin-18.
- •
ELISA: Enzyme-linked immunosorbent assay.
- •
AST: Aspartate aminotransferase.
- •
ALT: Alanine aminotransferase.
- •
ALP: Alkaline phosphatase.
- •
GDH: Glutamate dehydrogenase.