Drug-related hepatotoxicity is more common in renal transplant (RT) recipients with chronic liver disease because drug metabolism is not as efficient in these individuals. We describe a long-term survivor (30 years) of renal transplantation with hepatitis C virus (HCV) and drug-related hepatotoxicity. Our patient, a 26-year-old male, developed uremic syndrome in May 1976 and received a renal allograft from a related, living donor with an identical human leukocyte antigen genotype in August 1976. Maintenance immunosuppression treatment consisted of azathioprine (AZA) and prednisone. In 1993, the patient tested negative for HCV antibody v1.0 (anti-HCV). In 2000, the patient had elevated aminotransferases, which was attributed to pravastatin treatment. Remission of this abnormality was achieved once pravastatin was discontinued. In 2003, the patient again exhibited elevated levels of aminotransferases and AZA-related hepatotoxicity was suspected; therefore, AZA was discontinued and treatment with mycophenolate mofetil was initiated, which led to normal aminotransferase levels. The patient tested positive for anti-HCV v3.0 and HCV RNA and a liver biopsy showed chronic hepatitis with moderate activity. Currently, the patient’s renal transplant and liver are functional. In conclusion, hepatotoxic drugs should be used with caution in renal transplant recipients and close monitoring of liver function in patients with chronic viral hepatitis is crucial.
List of abbreviations
RT, renal transplant;
HCV, hepatitis C virus;
PDN, prednisone;
AZA, azathioprine;
anti-HCV, hepatitis C virus antibody test;
EIA, immunoenzymatic assay;
ALT, alanine aminotransferase;
MMF, mycophenolate mofetil;
HLA, human leukocyte antigens.
Concomitant use of specific drugs, such as immunosuppressive medications and statins, can have toxic effects on the liver in renal transplant (RT) recipients.1-4 Hepatitis C virus (HCV) infection is the main cause of chronic liver disease in RT recipients.1,5,6 Defining the long-term survival of RT recipients with HCV infection remains difficult for several reasons, such as the lengthy duration of the disease, difficulty in determining disease onset, coinfection with hepatitis B or human immunodeficiency virus, alcohol abuse, reluctance of nephrologists to perform liver biopsies, low aminotransferase levels, blunted humoral immune response (which makes antibodies harder to detect) and immunosuppressive therapy (which promotes viral replication and accelerates the progression of liver disease).1,7-12 HCV infection affects survival in RT recipients, mainly from the second decade post-transplantation onwards.11,13 Despite the absence of symptomatology and the subtlety of biochemical manifestations of HCV infection in RT recipients, histological evidence indicates that progression of the disease continues.8 The aim of this report is to document a case of drug-related hepatotoxicity in an RT recipient with long-term survival and HCV infection.
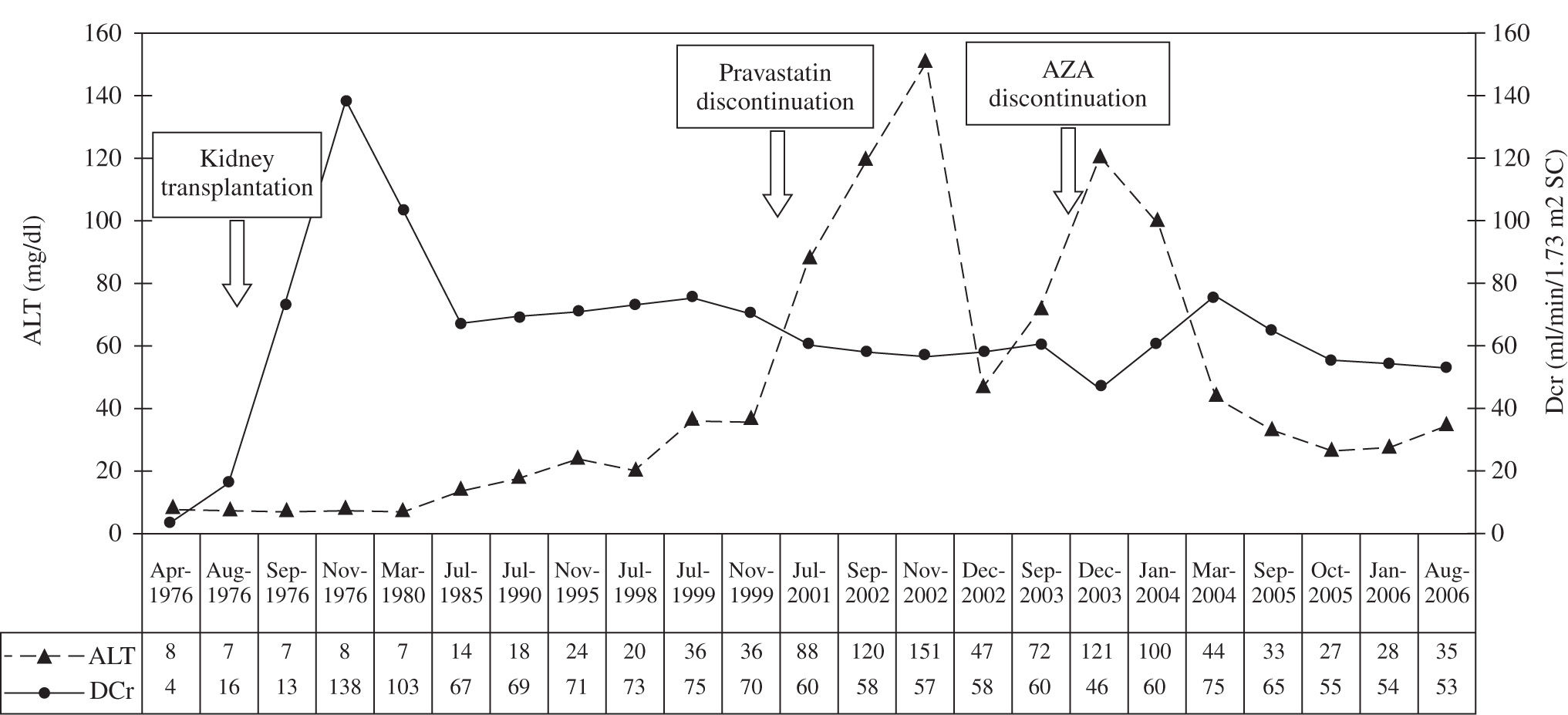
Case reportA 26-year-old man was diagnosed with end-stage renal disease in February 1976. He required multiple blood transfusions because of severe anemia. Laboratory results showed creatinine levels of 13.6 mg/dL and creatinine clearance of 3.7 mL/min (Figure 1). Ascendant pyelography demonstrated left renal agenesis and hemodialysis was performed. In August 1976, the patient underwent RT from a related living donor (sister) with an identical human leukocyte antigen (HLA) genotype. The perioperative immunosuppressive regimen included prednisone (PDN) at 90 mg/day and azathioprine (AZA) at 150 mg/ day, as well as cyclophosphamide at 200 mg/day and methylprednisolone at 375 mg/day. When the patient was discharged, the PDN dosage was tapered to 45 mg/day and the AZA dosage was tapered to 100 mg/day. Maintenance immunosuppressive therapy included AZA at 50 mg/day and PDN at 5 mg/day. Post-transplantation creatinine clearance was 73 mL/min (Figure 1). In 1993, the HCV antibody test (anti-HCV) by immunoenzymatic assay v1.0 (EIA) and hepatitis B surface antigen test were performed and were found to be negative. In March 1999, the patient was diagnosed with hypercholesterolemia (257 mg/dL) and was started on pravastatin at 10 mg/day. In 2000, he developed elevated alanine aminotransferase (ALT) levels and pravastatin was discontinued, which led to improvement of the transaminasemia. Cholesterol levels have since normalized. In 2003, ALT levels were elevated again and AZA was discontinued, which led to the initiation of mycophenolate mofetil (MMF) treatment at 500 mg/bid after which the transaminasemia normalized. PDN was continued at 5 mg/day.
Evolution of ALT and creatinine clearance in our renal transplant recipient from 1976 to 2006. The figure also shows that ALT levels decreased with the discontinuation of pravastatin and AZA. ALT indicates alanine aminotransferase; AZA indicates azathioprine; MMF indicates mycophenolate mofetil; DCr indicates creatinine clearance.
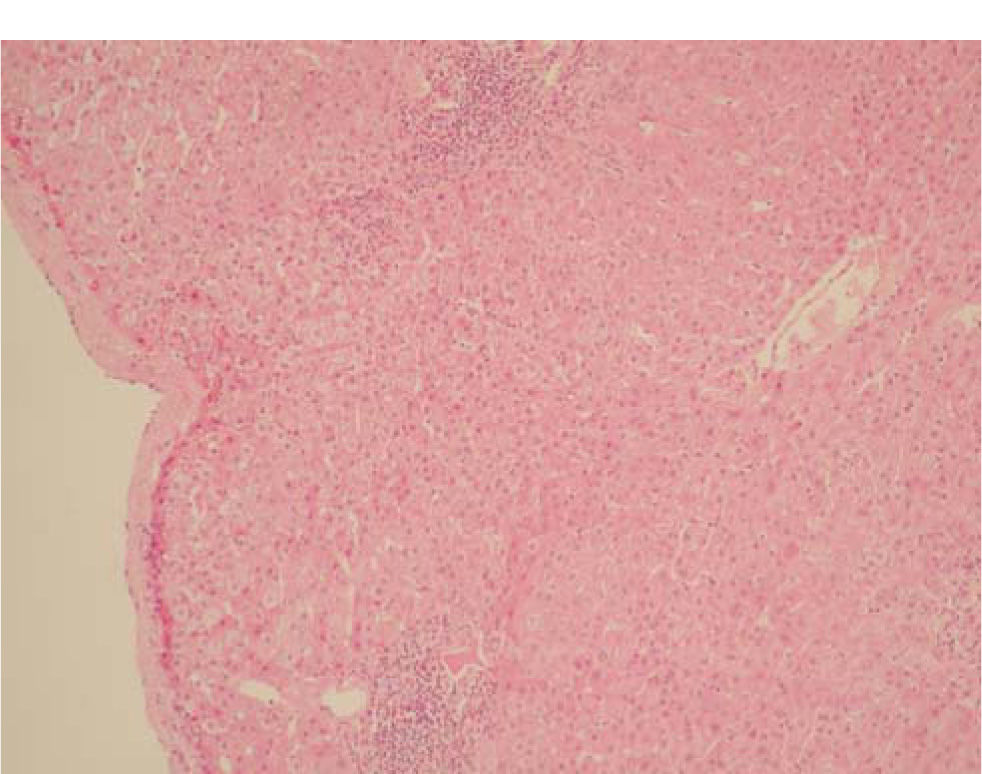
In December 2003, an EIA v3.0 test on the patient for anti-HCV was positive and a quantitative measurement of serum HCV RNA revealed levels of 498,000 IU/mL and genotype 1 a/b. A liver biopsy showed chronic hepatitis with moderate activity, lobular components, mild fibrosis and little damage to the ducts. Steatosis was not observed. The Metavir index of fibrosis was A2 F1 (Figures 2-4). The patient’s renal donor was negative for anti-HCV (EIA v3.0).
The patient had ceased alcohol consumption since the RT and had not manifested liver disease. The latest evaluation, in 2006, showed that the patient’s complete blood count, lipid profile, uric acid levels, full electrolyte profile, alkaline phosphatase, serum proteins, glucose, urinalysis, ALT and serum creatinine, were all normal. A hepatitis B surface-antigen test was negative, and a quantitative measurement of serum HCV RNA was > 700,000 IU/mL.
DiscussionThe purpose of this report is to document drug-related hepatotoxicity (pravastatin and AZA) and long-term survival (30 years) in a RT recipient with HCV who responded well to discontinuation of these drugs. The first renal transplant in Latin America was performed in Mexico at the Mexican Social Security Institute in 1963. Our case was the first RT recipient in Guadalajara, Mexico.14
The patient developed transaminasemia, and pravastatin was discontinued, which resulted in improvement of this abnormality. Although pravastatin is one of the safest statins available for use in transplant recipients, hepatotoxicity can develop in recipients with concomitant chronic liver disease.2,4 The patient’s second ALT elevation normalized once AZA was discontinued and MMF was initiated, supporting the diagnosis of AZA hepatotoxicity. AZA has been linked to adverse effects on the natural history of HCV infection in RT patients.3 RT recipients may develop AZA hepatitis despite the absence of any change in immunosuppressive regimen or kidney and liver function. New drugs, such as MMF, have displaced AZA as the cornerstone in immunosuppressive therapy for RT. MMF exerts potent intrinsic antiviral activities, reducing liver disease progression and, consequently, improving patient survival.1,8 Hepatotoxic drugs must be used with caution, and close monitoring of liver function in patients predisposed to chronic viral hepatitis is crucial.1-4 Despite HCV infection in our patient, normal aminotransferase levels have been achieved for more than two years since discontinuing both hepatotoxic drugs. Additionally, we have demonstrated that the patient has no hepatic insufficiency and that the RT remains functional.
Immunosuppression plays an important role in the progression of HCV infection in RT recipients; viremia levels rise 10-to 100-fold after RT.1,5,6,10,12 Reducing the dosage of the drugs or discontinuing the drugs are two strategies employed to limit the progression of liver disease.11 The timing of the infection is also important because patients who acquire HCV de novo, at or after transplantation, often have a rapidly progressive course of the disease. Patients with end-stage renal disease and HCV infection who have undergone RT show slower progression of liver disease.10,15 One limitation in our report is that only a single liver biopsy was available, restricting our description of the evolution of liver disease. In this case, it is difficult to estimate the exact infection date because no screening tests were available for HCV when the patient was transfused and began hemodialysis. Additionally, it is possible that the patient had a false negative anti-HCV v1.0 in 1993 because the reported rate of false negatives with EIA v1.0 was 15–40% in patients with renal damage. HCV infection was later demonstrated by anti-HCV with EIA v3.0, which has a sensitivity of 99%, and this was confirmed with an HCV RNA test.16
To our knowledge, our patient is one of the longestsurviving RT recipients reported worldwide and exemplifies the interactions between HCV infection, renal transplantation and hepatotoxic drugs. A major factor that influences survival in RT recipients is HLA compatibility. The advantage of matching HLA between transplant recipient and donor increases beyond the first post-transplant year. This is seen in an estimated half-life of 26.9 years for HLA-identical grafts, and 12.2 and 10.8 years for grafts from a sibling or a parent that are matched for one haplotype.17 Survival rate in HCV-positive RT patients is controversial. The general consensus is that long-term survival of HCV-positive RT patients is lower than in HCV-negative RT recipients.7 However, one report concluded that HCV infection does not influence patient or graft survival within a mid-term follow-up period in recipients of allografts from living donors.18 Furthermore, another report found that adjusted survival of HCV-positive patients is slightly superior to that of HCV-negative patients.19 It is important in our case that using an identical HLA renal donor allowed for lower doses of immunosuppressive drugs to be used, which reduced adverse effects and resulted in only two events of hepatotoxicity in 30 years of follow-up.
HCV infection requires treatment to limit disease progression and prolong survival. Currently, treatment involves a combination of pegylated interferon and ribavirin. However, in RT recipients with HCV infection, interferon places the patients at high risk for allograft rejection and graft loss. Presently, there is no effective therapy for this group of patients.11,20
Our report supports previous studies reporting that HCV is not a contraindication to RT,10,18 because it demonstrates one of the longest surviving (30 years) cases reported for a kidney transplant recipient and the recipient has HCV.
AcknowledgmentsWe are indebted to Pedro Kristian Rivera-Aguilar MD, Daniel I. Arroyo-Espinosa MD and José M. Mendez-Ramirez MD for their contributions to the manuscript.