Introduction. Hepatitis A virus can evolve to acute liver failure with a fatal outcome if it is not reversed.
Objective. We describe the clinical course of 12 children who presented with hepatitis A acute liver failure and received treatment with oral N-acetylcysteine (NAC).
Materials and methods. Of the seventy-two patients with viral hepatitis A, 12 patients who had acute hepatic failure were included. The variables evaluated were age, sex, duration of clinical features prior to hospitalization, signs and symptoms, laboratory parameters [alanine aminotransferase (ALT), aspartate aminotransferase (AST), prothrombin time (PT), partial thromboplastin time (PTT), internal normalization ratio and ammonia], treatment (oral NAC 100 mg/kg/day, lactulose, neomycin and general measures) and clinical course during hospitalization.
Results. Six males and six females were included. School-aged and adolescent children predominated. All presented with jaundice, nausea, vomiting and hepatomegaly. Two had stage 2 neurological signs as per the West-Haven scale. All had altered laboratory parameters. All received NAC, six patients for a week and the remaining six for 9-36 days. Treatment was not ceased until patients showed clinical and laboratory improvement. All data were analyzed using both student’s t test and Wilcoxon signed rank with alpha = 0.05, the ALT with P = 0.0003 and 0.005, AST with P = 0.0001 and 0.0005, PT with P = 0.0237 and 0.0005, PTT with P = 0.0515 and 0.0039, ammonia with P = 0.0197 and 0.0015 and direct bilirubin with P = 0.0190 and 0.068. There was good tolerance to medications and a satisfactory clinical course.
Discussion. The use of oral NAC appears to be an effective therapeutic alternative for hepatitis A-induced liver failure if it is offered appropriately. It can modify the clinical course to a favorable one and prevent the fatal outcome of hepatic encephalopathy.
The hepatitis A virus belongs to the Picornaviridae family and is probably the most frequent cause of acute liver failure (ALF) in Latin America.1-3 In the pediatric age group, the course of the disease in many children can be unapparent because it occurs in its anicteric form, although in school-aged and adolescent children it usually manifests with more obvious signs and symptoms.4-6
The number of patients with viral hepatitis A who develop liver failure and a fulminant form of hepatic encephalopathy (HE) is not precisely known. In general, it has been reported that 26% of ALF has a hepatitis A etiology, and in the registers of transplant centers, 10% of acute hepatic failure has the same etiology. Fifteen to twenty-five percent of all cases of ALF are secondary to infection by hepatitis A virus.4 The major clinical signs are malaise, weakness, nausea, vomiting, abdominal pain and progressive jaundice. Some patients can develop ALF without jaundice. The laboratory changes that are associated with ALF are altered coagulation tests, increase in serum ammonia and hypoglycemia. If hepatic failure is not reversed, the patient invariably develops HE, a dreadful complication associated with a high mortality.3-8
For the past 36 years, the Child-Pugh classification9 has been used to scale the severity of hepatic disease, and more recently the Model for End Stage Liver Disease10 has been used to evaluate the functional hepatic capacity in those patients who are selected for hepatic transplant. However, the scores obtained do not always coincide with progression of liver failure, especially in children. Therefore, it is prudent to also consider the clinical signs and the early changes in laboratory parameters such as alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels, prothrombin time (PT), partial thromboplastin time (PTT), internally normalized ratio (INR), ammonia, direct bilirubin, glucose, albumin level and, whenever possible, factor VII assay, to assess the progression and severity of the condition. With respect to treatment, it has been recommended that the earlier the clinical alterations associated with hepatic failure are identified, the greater the possibility that the patient will overcome liver failure without developing HE. The therapeutic alternatives available are prostaglandin E, L-ornithine, N-acetylcysteine (NAC), molecular absorption recirculating system, plasmapheresis and hepatic transplant.4,11-19
In this article, we describe the results obtained with use of oral NAC together with lactulose, neomycin and other supportive measures as a therapeutic regimen, and follow the recovery of an adolescent who had HE secondary to hepatitis A who was treated with the aforementioned regimen.11
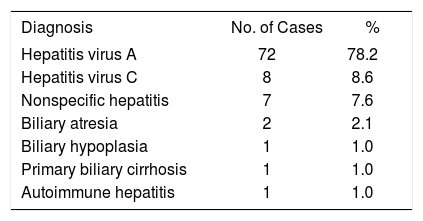
Materials and MethodsDuring the period from January 2000 to December 2008, 92 cases of hepatitis of different etiologies were hospitalized, among which 72 (78.2%) were caused by hepatitis A (Table 1). In these 72 cases of hepatitis A, 20 patients developed complications, 10 ALF and 10 HE, and eight patients died during the course of their hospital stay. In this study, we report only the 12 cases treated with NAC that we consider were improved as assessed by clinical and laboratory parameters.
Established final diagnosis in 95 patients categorized with initial diagnosis of hepatitis.
| Diagnosis | No. of Cases | % |
|---|---|---|
| Hepatitis virus A | 72 | 78.2 |
| Hepatitis virus C | 8 | 8.6 |
| Nonspecific hepatitis | 7 | 7.6 |
| Biliary atresia | 2 | 2.1 |
| Biliary hypoplasia | 1 | 1.0 |
| Primary biliary cirrhosis | 1 | 1.0 |
| Autoimmune hepatitis | 1 | 1.0 |
Since 1997, a therapeutic scheme has been used at the State of Sonora Children’s Hospital that includes oral NAC with lactulose and neomycin as treatment in children with ALF and HE of different etiologies. After the successful recovery of an adolescent with HE secondary to hepatitis A who was treated with these drugs, we decided to utilize the same scheme for consecutive patients. The criteria for ALF were absence of chronic hepatic disease, clinical and serological markers of hepatitis A, prolonged prothrombin time, INR > 1.5, poor response to intravenous, application of vitamin K, elevated transaminases and serum ammonia, and patients with or without neuropsychiatric signs. Our therapeutic regimen included NAC 100 mg/kg orally or by nasogastric tube every 4 h for the first 16 h, then the same dose continued at intervals of 6-8 h depending on the clinical response and results of laboratory monitoring of ALT, AST, PT, PTT, INR, bilirubin and ammonia every 12-24 h. Depending upon the clinical course, NAC was continued as long as required for normalization of laboratory parameters. Other treatment included lactulose 10 g orally every 6 h and 30% lactulose solution as an enema (using the powder presentation for dilution), oral neomycin at 50 mg/kg/day and 2% neomycin by means of enema every 12 h. These drugs were administered at the same time as NAC and were suspended once the hepatic enzyme levels, coagulation profile and ammonia level started to improve. The dietary protein intake was restricted to 0.5-1 g/kg/day. The diet was provided orally or by nasogastric tube depending upon the patient’s tolerance. When necessary, the patients were fasted for a period and maintained with mixed parenteral nutrition of 10% dextrose solution. Other supportive measures given according to the patients’ needs were: strict fluid balance, correction of acid base and electrolyte imbalance, vitamin K, fresh plasma, platelet concentrate and fresh whole blood. This treatment was used in 18 children who presented with ALF and HE. All children who were admitted with features of ALF during the 8-year period were included in the study. This analysis is a retrospective study based on the experience obtained with this treatment modality and therefore does not have the characteristics of either controlled or double blind studies.
The variables analyzed were age, sex, duration of course prior to admission, signs and symptoms, neurological signs according to the West-Haven Scale,4 duration of hospitalization, altered laboratory parameters [transaminases (ALT and AST), alkaline phosphatase, bilirubin, serology for hepatitis A virus, glycemia, urea, creatinine, PT, PTT, INR, platelets, protein, albumin, serum ammonia and culture], treatments (NAC either per oral or nasogastric route and lactulose and neomycin by oral and rectal route), tolerance to medications, duration of administration and time taken for normalization of laboratory parameters, use of ursodeoxycholic acid and duration of clinical course. Statistical analysis was performed with the statistical package JMP SAS.20
ResultsThe age distribution of the patients was as follows: two were between 5 and 7 years (16%), six were between 8 and 11 years (50%) and four were between 15 and 17 years (33%). Six were female and six were male. The duration of the clinical course before hospital admission varied between 4 days and 1 month. Seven patients (58%) had a duration between 4 and 7 days, four (33%) between 9 and 15 days and one patient between 16 days and 1 month, with an overall average of 10.6 days. The signs and symptoms in all the patients were anorexia, nausea, vomiting, jaundice, hepatalgia and hepatomegaly. Four had epistaxis and ecchymosis (Table 2).
Of the 72 cases of hepatitis A, 20 patients developed complications. Ten presented with ALF and in addition 10 developed HE. Of these, two were detected early in stage 2 HE as defined by the West-Haven Scale; four presented with neurological manifestations of West-Haven Scale stages 3 and 4 at the time of admission and the other two cases presented with ALF and developed HE during their hospitalization.
Among the laboratory studies, urine general examination, urea, creatinine and glycemia tests were within normal limits. In two children, serum albumin was less than 3 g/l and platelets were less than 150,000 x 103/mL in two children. The serological tests for hepatitis A virus (IgG and IgM), processed by immunoenzymatic methods, were positive in all patients.
ALT and AST varied from 60 to 4,817 IU and 156 to 4,560 IU, respectively. Only one patient who had lower levels of enzymes at the time of admission subsequently had elevated levels that required modification of their treatment. PT and INR were abnormal in all cases: in three children, they were initially within normal limits but were altered 4 days after admission. PTT was abnormal in eight cases. Serum ammonia levels were found to be well elevated in nine patients, three of whom had PT and PTT within normal limits on their day of admission.
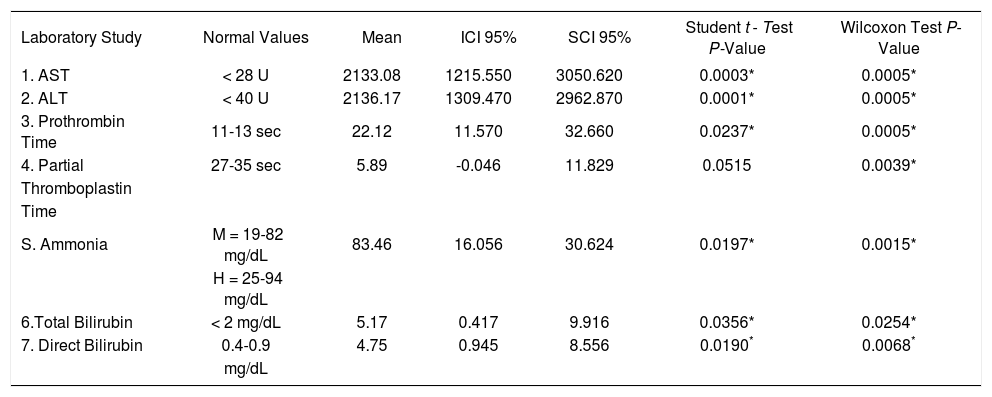
Because the patients showed clinical improvement, the laboratory constants used for follow up were analyzed by paired Student t test and Wilcoxon’s test for the nonnormally distributed values and showed significant changes. There were similar changes in ALT and AST in all 12 patients, including the two who presented with neurological changes. The levels of total and direct bilirubin as well as the results of coagulation profiles such as PT, PTT and INR also improved (Table 3).
Changes in the laboratory constants. N = 12.
| Laboratory Study | Normal Values | Mean | ICI 95% | SCI 95% | Student t - Test P-Value | Wilcoxon Test P-Value |
|---|---|---|---|---|---|---|
| 1. AST | < 28 U | 2133.08 | 1215.550 | 3050.620 | 0.0003* | 0.0005* |
| 2. ALT | < 40 U | 2136.17 | 1309.470 | 2962.870 | 0.0001* | 0.0005* |
| 3. Prothrombin Time | 11-13 sec | 22.12 | 11.570 | 32.660 | 0.0237* | 0.0005* |
| 4. Partial | 27-35 sec | 5.89 | -0.046 | 11.829 | 0.0515 | 0.0039* |
| Thromboplastin | ||||||
| Time | ||||||
| S. Ammonia | M = 19-82 mg/dL | 83.46 | 16.056 | 30.624 | 0.0197* | 0.0015* |
| H = 25-94 mg/dL | ||||||
| 6.Total Bilirubin | < 2 mg/dL | 5.17 | 0.417 | 9.916 | 0.0356* | 0.0254* |
| 7. Direct Bilirubin | 0.4-0.9 | 4.75 | 0.945 | 8.556 | 0.0190* | 0.0068* |
| mg/dL |
Of the 12 patients who received NAC, nine patients received it orally with good tolerance and the other three patients required nasogastric tubes because they presented with nausea and vomiting. None reported any side effects such as gastric irritation or cutaneous rash. The lactulose and neomycin were administered orally as well as by enema in eight patients. The average duration of treatment was 15 days. In one patient, treatment was extended for an additional 15 days with an NAC dose of 25 mg/kg/day until ALT and AST levels returned to normal, but lactulose and neomycin were suspended at the time of discharge.
The remarkable manifestation of cholestasis in two patients with direct bilirubin of 13.6 mg/dL and 19.4 mg/dL deserves special mention. Oral ursodeoxycholic acid at 15 mg/kg/day was used, followed by a notable reduction in direct bilirubin levels within 6-9 days compared with two other patients with marked cholestasis in whom it took up to 18 days to reach normal bilirubin levels.
Considering the response to treatment with NAC, the time taken for normalization of laboratory values in half the patients was 8 days. In four cases it took 10-15 days and the remaining two cases needed 30 days. The average duration of hospitalization was 12.7 days.
Among the 20 children with complications, 18 received treatment with NAC. Twelve cases progressed in a satisfactory manner. Eight patients died, six of whom presented with encephalopathy. Four patients passed away during the first 7-12 days, one case on the 19th day and one 60 days after hospital admission. The clinical course of these patients is described in a separate study in which other types of hepatopathy and EH were analyzed.
DiscussionIn the nineties, complications due to viral hepatitis were estimated to occur in between 0.2% and 0.4% of symptomatic cases, and were considered to be more frequent in adults than in the pediatric age group.21 Recently, however, reports from hepatic transplant centers suggest that 26% of cases with ALF are caused by hepatitis A.3,4 In Mexico, in our epidemiologic surveillance system, 388,870 cases were registered between 1990 and 2007. Of these, 81.4% were related to hepatitis A virus, 6.7% to hepatitis B or C virus and 12% to other types of hepatitis. Unfortunately, the mortality rate of hepatitis A infection is not known and it is not considered a public health problem, because this infection occurs mainly in the first few years of life and has been categorized as a benign disease.1,2
In our hospital, as shown in Table 1, hepatitis A constitutes the most frequent cause of hospitalization for hepatitis. Complications occurred in 20 of 72 (27%) hospitalized patients. Eight of these patients died (11%), which is not a low value. On the other hand, the death rate among those who developed ALF and EH was also high.
In search of an accessible therapeutic resource, we selected NAC as a proven drug because of its effectiveness especially in patients with acetaminophen poisoning. In our setting, because of the unavailability of the intravenous form of NAC, we decided to give the drug by nasogastric tube in an adolescent with HE secondary to hepatitis A.11 Encouraged by the clinical course of this patient, we chose to use NAC continuously and subsequently included each new case of ALF caused by hepatitis A. The therapy was initiated in an appropriate way. The drugs were administered for 48-72 h, longer than the traditionally recommended time, based on the possibility of providing hepatoprotection for a greater period. Treatment was continued until normalization of laboratory parameters, at the same time providing measures to diminish the increased levels of ammonia.4,14,22-27
More recently, there have been case reports of patients with ALF of etiology other than acetaminophen poisoning that have been treated with intravenous NAC. The most recent study in June of 2009 concluded that NAC improves transplant-free survival in patients with early stage nonacetaminophen-related ALF, but patients with advanced coma grades do not benefit.19,24,27
The clinical basis for the use of NAC is based on diverse mechanisms, of which the most important are:
- a)
To facilitate the synthesis of depleted glutathione in ALF
- b)
To serve as a specific substrate for hepatic microsomal glutathione transferase, thereby improving oxygenation.23
- c)
Increasing the blood flow by increasing the soluble nitric oxide activity in the glutamyl cyclase system
- d)
- e)
NAC has been utilized in an experimental form, encapsulated in liposomes, in laboratory animals, where it confers hepatoprotection against the toxic effects of lipopolysaccharides.34
To date, little is known about the optimum duration of administration of NAC. It is up to the doctor to make an individual determination in a practical manner,25-29,35 although it has been suggested for more than 15 years that it can be used for more than 3 days.19-25
Of the 12 cases reported here, the duration of administration of NAC was between 10 days and 4 weeks in six children, without observing any undesirable side effects. Because we are dealing with a retrospective, descriptive study, in which the number of days needed for treatment was less than 9 days in 50% of the patients while the remaining 50% required 10-36 days treatment, it is not possible to establish a recommendation for an appropriate time required for administration of oral NAC. However, we were able to observe clinical improvement in 12 patients, similar to the earlier published study regarding the use of NAC in a patient with nonacetaminophen-induced ALF.19,24,27 It is possible that our opportunistic use of oral NAC was beneficial, as evidenced by the improvement in laboratory values as shown in Table 3. Without pretending that our experience suggests substituting oral for intravenous use of NAC,4,24,27 oral administration seems to be an acceptable option especially in patients with ALF who are identified early, even in those who manifest with early neurological signs.4,24-29
In relation to the stage at which ALF was identified, seven of the 12 patients corresponded to the hyperacute phase and five to the acute phase,26 with only two patients presenting with West-Haven Scale stage 2 neurological signs, signifying a better prognosis.3,4,15-17,24 It is strongly recommended that the general physician and pediatrician consider the necessity of monitoring the laboratory parameters from the beginning of the disease and for a minimum of 2 weeks. In this way, it is possible to intervene in the early stages of disease.4,15-17,30,31
With respect to the use of ursodeoxycholic acid in patients of viral hepatitis A with intense cholestasis, we used it in two patients with a good response. This drug is suggested for blocking oxidative stress, thus avoiding the accentuation of hepatic damage, and for its hydrophilic and choleretic effect in displacing the toxic bile acids.36
In our country, according to the health system registry,1 it is likely that a greater number of cases caused by viral hepatitis A with fulminant evolution and mortality exist as described in the previous part of this article. Hence, it is extremely important that vaccination against the hepatitis A virus should be considered within the National Immunization Scheme of Mexico, so that we will not have to regret more deaths from this cause.1-3,37
AcknowledgementThe Authors would like to acknowledge Ignacio Fonseca Chon, M.Sc., for his statistical advice.