Livers cold preserved during variable periods of ischemia suffer functional, morphological and hemodynamic alteration, which are exacerbated when they are reperfused. One important injury is glycogen depletion during cold ischemia/ reperfusion. How liver can restore their energy during reperfusion is related with the preservation time, nutritional status of the donor, and the preservation solution used. However, there are some treatments that help livers to preserve their energy storage. These procedures used drugs or metabolites, which are added to the liver to maintain their glycogen storage during preservation, time and allow the organ to restore its energy during reperfusion. There are several publications where the nutritional status of the donor was studied. There is controversy about the quality or the donor organ. Some authors say that fasted animals are better donors because this condition reduced hepatic injuries; others think that fed animals provide the necessary glycogen (energy) to improve liver preservation, reducing morphological and functional damages. Many others are convinced that nutritional status of the donor is not relevant because hepatic injuries will occurred even though the donor was fed or not. The preservation solution has an important role in reducing liver damages during cold ischemia/reperfusion and in restoring liver energy storage. Storage in HLR (histidine, lactobionate and raffinose) solution facilitated the resuscitation of energetic status and preserved adenine nucleotide levels significantly greater than Marshall’s citrate or Bretschneider’s histidine-based solution (HTK). University of Wisconsin (UW) proved to be suitable for energy recovery during reperfusion. In conclusion, the aim of this review is to present studies performed by different authors where they analyzed preservation/reperfusion injuries, how the liver restores its energy storage during reperfusion time, different strategies to avoid glycogen depletion during cold ischemia/reperfusion, the efficacy of preservation solutions and the effect of nutritional status of the donor to prevent functional alteration of the liver during cold preservation.
Abbreviations:
HLR (histidine, lactobionate and raffinose) solution
HTK: Bretschneider’s histidine-based solution
UW: University of Wisconsin
ATP: adenosine triphosphate
ADP: adenosine diphosphate
AMP: adenosine monophosphate
db-cAMP: dibutyryl-cyclic adenosine monophosphate
AMPK: adenosine monophospate-activated protein kinase
HC–Marshall’s citrate: hypertonic citrate solution
TA: adenine nucleotide levels
EC: energy charge
PAS: Peryodic Acid Schiff
J.V.R and E.E.G. are members of The National Council of Research (CONICET), Argentina.
This study was supported by Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) Argentina, PICT 05-06434, BID 1201 OC/AR.
IntroductionReperfusion gof livers exposed to variable ischemia time period during hepatic surgery can cause severe injury to hepatic tissue.1 Direct effects of cold ischemia on livers produces cell swelling, interstitial edema, denudation of the sinusoidal lining cells by alterations in connections between cells and extracellular matrix2,3 and activation of proteases.4,5 Extracellular matrix damages cause changes on the morphology of parenchymal and non-parenchymal cells. In addition, hepatic injury is further aggravated by disturbances of hepatic microvascular blood flow in the post ischemic period that may be a consequence of nonparenchymal cell damage. These alterations increase vascular resistance, impeding the appropriate oxygen transport and delivery of nutrients from blood to hepatocytes. This phenomenon always precedes hepatocyte injury, suggesting that abnormalities in microcirculation could play a primary role in the pathogenesis of the graft non function.6
The clinical experience indicates that poor graft function might be related to additional factors. Simple cold storage of livers for transplantation activates glycolysis due to lack of oxygen. Energy derived from glycolysis may be critical for cell survival and liver cell death may occur once glycolysis is inhibited in the liver due to accumulation of end products or lack of substrates (glycogen).7 Pre-harvesting conditions of the donor have influence on glycogen content and graft function and survival after preservation/reperfusion period.8
Experimentally, the nutritional status of the donor is known to influence the glycolitic support of adenine nucleotide pools in ex-vivo-preserved livers. Pretreatment of donors with glucose effectively retards hepatocellular ATP depletion in preserved livers by potentiation of their glycolitic capacity.9 Many studies have suggested that glycogen in donor livers is an important fuel during cold ischemic time and at reperfusion. However, it remains unclear as to whether the depression of glycogen content in the graft results in a critical derangement of energy metabolism after reperfusion.10 Practically, it is almost impossible to pretreat donors to obtain his best nutritional status before transplant since most of them are from cadaveric donors.
Preservation solutions might contribute in different way to restore the energy during reperfusion.11 Despite the advances provided by University of Wisconsin (UW) solution in the field of liver preservation, primary graft dysfunction and non-function remain challenging in the early postoperative course following liver transplantation. To date, no definite causal factors have been identified in the pathogenesis of this complication, and routine liver function tests have not proved to be reliable in predicting graft outcome. However, the nutritional status of the liver as mentioned above has been proposed as an important factor.9
The purpose of this review is to analyze hepatic energetic alterations due to cold storage and different strategies to avoid hepatic glycogen depletion during cold preservation/reperfusion time.
Preservation/reperfusion injuriesThe nutritional status of the donor influences the glycolitic support of adenine nucleotide pools in ex-vivo-preserved livers. In MNR spectroscopic studies in the mouse liver, it was demonstrated that the intrahepatic glycogen decreased significantly after 6 Hs of preservation, independent of which preservation solution is used, suggesting that glycogen is used as an energetic substrate during cold ischemia.9 Many studies of human donor livers indicate an association between ex vivo hepatocellular adenosine triphosphate (ATP) and post transplant graft function. One clinical study of transplant recipients revealed a direct association between satisfactory energy status of donor livers and successful postoperative allograft function. The livers that functioned well after transplantation have both higher levels of ATP and adenylate energy charge (the ratio [ATP + 0.5 ADP] / [ATP + ADP + AMP], where ADP is adenosine diphosphate and AMP is adenosine monophosphate) than those livers that subsequently failed.12 Another clinical study indicated that post transplant allograft function correlated with total adenine nucleotide levels at the end of the hypothermic preservation period.13
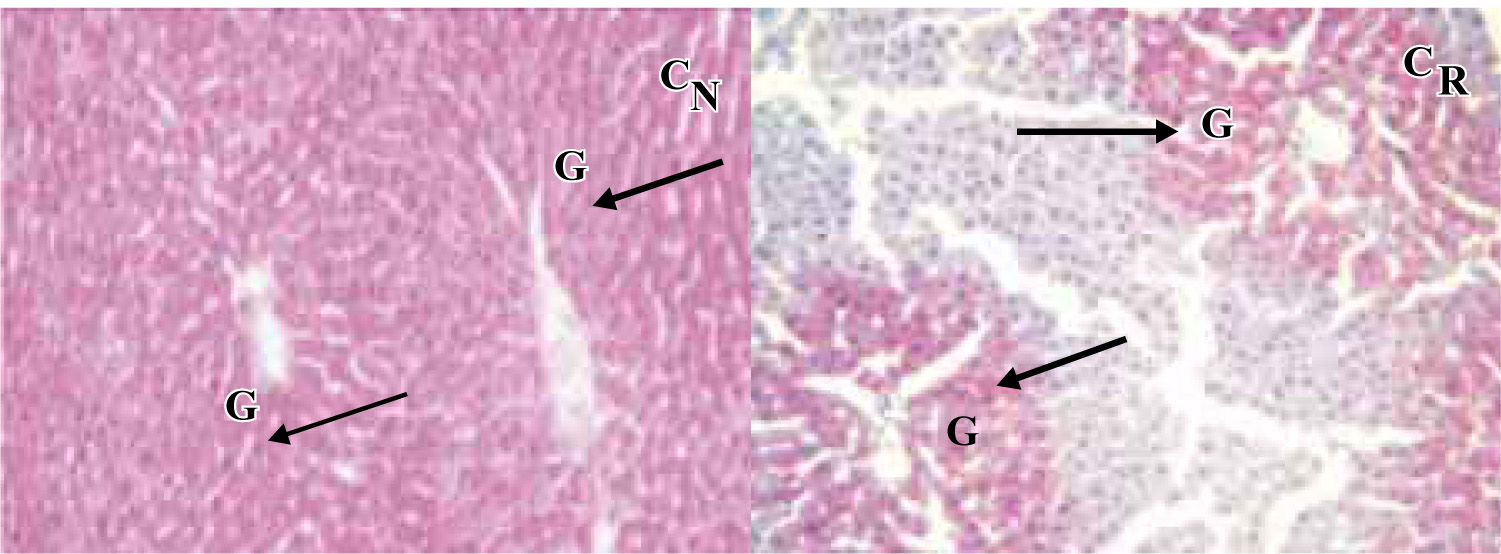
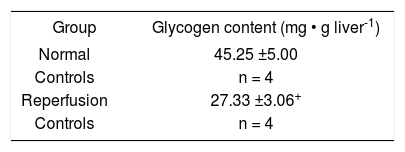
Our experience with rat livers cold preserved during 48 Hs in a modified UW solution, demonstrated that glycogen content was dramatically reduced during reperfusion14 even though donors were fed until surgery (Table I,Figure 1).
There are many studies were authors analyzed glycogen loss after cold ischemia and reperfusion. Cherid et al., evaluated 62 surgical biopsies for the loss of glycogen and its variations in relation to cold ischemia, reperfusion, lobular zonation and donor’s ages. They found a clear lobular zonation of glycogen during the cold ischemia and reperfusion steps. In periportal areas they established a diminution of glycogen content in a range of 48% and 74% in pericentral ones during cold ischemia. These percentages increased after reperfusion (60% in periportal areas and 95% in pericentrilobular ones).15
Dodero F. et al., used histochemical quantitative analysis to examine glucose metabolism in liver grafts after cold ischemia and reperfusion. They found a heterogeneous lobular distribution pattern of glycogen content. In most of the cases, this heterogeneous pattern of glycogen was observed after preservation and reperfusion. However, a 42% reduction of glycogen content, expressed as the ratio between stained surface and total surface of liver biopsies, was observed after reperfusion. Both periportal and centrilobular hepatocytes showed a significant decrease in mean optical density after reperfusion (18% and 25%, respectively).16 Glucose-6-phosphatase activity was also studied. This enzyme catalyzes de enzymatic dephosphorylation of D-glucosa-6-fostato to restore free D-glucose released into the blood; this reaction occurs only in the liver. The activity of this enzyme was maintained after reperfusion in most of the cases by periportal hepatocytes. In centrilobular ones, more cases showed a decrease in enzyme activity. It is suggested that ischemia-reperfusion mainly affects the glycogen content in both periportal and centrilobular hepatocytes and that centrilobular glucose-6-phosphatase activity is more sensitive to ischemia-reperfusion injury than the one in periportal hepatocytes.17
Restoration of liver energy during cold preservation and reperfusion periodsTsukamoto K, established that functional recovery of the preserved organ depends on the recovery of energy metabolism. To assess this recovery, ATP level and tissue pH were measured by phosphorus nuclear magnetic resonance. Oxygen consumption, bile flow rate and glucose output were also measured before and after preservation in Euro-Collins solution during 6 or 24 Hs at 0ºC. Livers preserved during 6 Hs recovered 92% of ATP level of the pre-preservation level, and the ones preserved during 24 Hs only 76%. Tissue pH recovered the pre-preservation level after 6 and 24 Hs preservation. Oxygen consumption of the 24 Hs preserved liver was higher than that of the fresh liver. These findings support the idea that the efficiency of oxidative phosphorylation decreases after 24 Hs preservation, but still remains the possibility of increase in ATP hydrolysis by other processes than bile secretion.18
Churchill TA and Fuller BJ, examined the effects of dibutyryl-cyclic adenosine monophosphate (db-cAMP) and okadaic acid (a specific inhibitor of protein phosphatases 1 and 2A) as additives to a cold storage solution. These studies were applied on rat livers cold preserved during 24 Hs. They studied the effects on levels of glycogen phosphorylase, the resultant effects on flux through the glycolitic pathway, and the consequences of these changes on adenylate (ATP, ADP and AMP) levels. They found that glycogen phosphorylase and not necessarily glycogen content is one major determinant in maintaining anaerobic metabolism and energy production during cold liver storage.19
Miki et al., determined the glycogen content of 28 donor livers and the plasmatic concentration of some metabolic substrates measured during liver transplantation. Gluconeogenesis was maintained even in the glycogen-depleted graft during reperfusion. However, glycogen-depleted grafts produced more ketone bodies until 24 Hs after reperfusion. Free carnitine concentrations in these patients were significantly higher than those in patients with glycogen-non depleted grafts until 48 Hs after reperfusion. This increment in carnitine concentrations suggested that glycogen-depleted liver graft might restore essential metabolic function by producing energy substrates through enhanced ketogenesis in the post reperfusion period. The enhanced production of carnitine by the graft provides a substrate for the production of ketone bodies and thus may be relevant to the enhanced ketogenesis.10
Strategies to prevent glycogen depletion during preservation/reperfusion periodLiver ischemic preconditioningIschemic preconditioning renders the liver more tolerant to ischemia-reperfusion injury in warm and cold ischemia-reperfusion models.
Ischemia-reperfusion injury (IR) is a serious problem in clinical transplantation; it is the second greatest cause of organ failure after immunologic graft rejection. One of these injuries is energy degradation during ischemia.
Ischemia preconditioning is an adaptive pathophysiological phenomenon, which consists in the application of brief and repetitive episodes of ischemia-reperfusion before a sustained IR.
Liver preconditioning minimizes the energy metabolism degradation occurring during sustained ischemia. This phenomenon is due to enhanced activity of adenosine monophospate-activated protein kinase (AMPK), which preserves ATP and reduces lactate accumulation during ischemia.20
Liver pre-treatment with metabolites or drugsAdenine nucleotidesPost transplant functional viability of the allograft liver is dependent upon its capacity to regenerate ATP for protein synthesis, ureagenesis, bile production, and restoration of ion gradients. A reduction in ATP synthetic capacity coupled with poor oxygenation during reflow could exacerbate parenchymal and nonparenchymal cell damage. Exogenous adenosine is an effective substrate capable of augmenting or supporting hepatocellular ATP concentrations in vivo or in vitro.21
Palombo et al, used the isolated perfused rat liver model to asses adenine nucleotide recovery following preservation in UW solution during 20 Hs. They added adenosine during reperfusion to enhance adenine nucleotide restoration. ATP and total adenine nucleotide content of livers perfused with an oxygenated Krebs/fluorocarbon solution containing 1 mM adenosine were restored to in vivo levels within 30 min of perfusion. Adenine nucleotide recovery in livers perfused without adenosine was only 65 % of control levels. Provision of supplemental adenosine to the allograft liver during initial reflow appears warranted to promote full and rapid restoration of adenine nucleotide content following extended preservation ex vivo.21
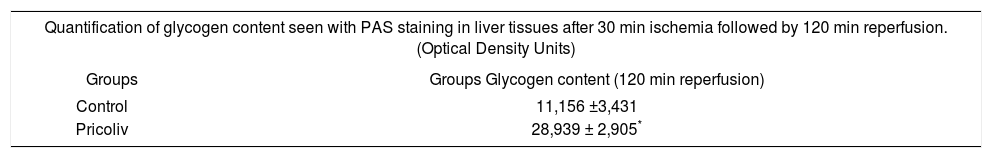
PicrolivPicroliv is a potent antioxidant derived from the plant Picrorhiza Kurrooa. It is basically a mixture of two iridoid glycosides, picroside-1 and kutkoside (1:1.5 w/w) and has been shown to provide significant hepatoprotective activities by modulation of free radical induced lipid peroxidation in in vitro systems. Pricroliv also modulates the expression of hypoxia inducible genes during hipoxiareoxygenation in different cells and efficiently regulates the expression of insulin like growth factor-1 and its receptor in cerebral hipoxia. Rats were fed with pricoliv in a dose of 12 mg/kg once daily by oral gavage for 7 days prior to hepatic ischemia. This pre-treatment resulted in better hepatocyte glycogen preservation (Table II, adapted from Singh et al.) and reduced apoptosis.22
Hepatoprotective activity of Pricroliv: * p < 0.05 compared with control rats.
| Quantification of glycogen content seen with PAS staining in liver tissues after 30 min ischemia followed by 120 min reperfusion. (Optical Density Units) | |
|---|---|
| Groups | Groups Glycogen content (120 min reperfusion) |
| Control | 11,156 ±3,431 |
| Pricoliv | 28,939 ± 2,905* |
Norepinephrine induces changes in energy metabolism in livers perfuse immediately after dissection and in livers stored in Euro-Collins solution at 0ºC for 24 Hs. In fresh liver, norepinephrine (1 μM) induced an increment of glucose in two-fold and oxygen consumption. Changes in ATP content and tissue pH were minimal. In stored liver, the cumulative increments in glucose output and oxygen consumption induced by norepinephrine were 30 and 27% those of fresh liver, respectively. Unstimulated liver has an ATP content of 76% that of the fresh one, then decreased to 50% during stimulation. A transient decrease in tissue pH was significant compared with that of fresh liver.23
In conclusion, norepinephrine stimulation is useful for energetic and functional recovery of liver preserved under hypothermic conditions and then reperfused.
Nutritional status of the donor and its effect on glycogen loss during preservation/reperfusion period.It has been suggested that depletion of donor hepatic glycogen reserves deleteriously affects the resistance of the hepatic graft to ischemic episodes.24-26 There is controversy over how the nutritional condition of the donor liver affects transplant outcome. Some studies suggest that livers from fasted animals (liver glycogen-depleted) are more injured than livers from fed animals. Studies were donor rats were fed and supplied with glucose during two or four days before surgery, shown more sensitive to cold and ischemia than fasted ones.27 Another study demonstrates that fed animals supplied with glucose, released less transaminases to the perfusate during reperfusion using the isolated and perfused liver model. Besides, they has fewer platelets than fasted animals; this indicates that hepatocytes and endothelial cells from fed animals suffered less injuries during cold preservation.28
Some authors proposed that the nutritional status of the donor has no influence in the prevention of ischemia/reperfusion injuries.29
One of the studies that support the idea that fasted rats are less vulnerably to ischemia than fed rats was performed by Sumimoto R et al., where they compared three groups of rats: fed, fasted (4 days) and fasted (2 days) and then feeding them only with glucose to elevate liver glycogen. Livers were preserved for either 30 or 44 Hs in UW solution and transplanted. Four-day fasting of the donor improved the survival rate in liver transplantation (50%-100% in 30–Hour cold storage, 29%-83% in 44-Hour cold storage). However, feeding glucose for 2 days to fasted animals caused a decrease in survival in this series of transplants. In the glucose-fed group, liver glycogen was 240% of that in the control group. This suggests that the presence of a high concentration of liver glycogen is not beneficial to the preserved and then transplanted rat liver.27
It has been propose that the nutritional status of the donor affect the outcome of liver transplantation by the inhibition of Kupffer cell which induced injury to the reperfused organ that leads to an inflammatory type response. This occurred by altering rats diet by either fasting or by feeding an essential fatty acid free diet for two months. This type of diet has been shown to reduce significantly the inflammatory response in rats.30
In 1988, Palombo et al., administrated intravenous dextrose to rats before organ procurement. This provision of glucose may enhance the capacity of the liver to generate energy by anaerobic glycolysis. They characterized the fed vs fasted state measuring liver glycogen, and plasma glucose, insulin, and glucagon concentrations. Respectively, mean glucose levels were 169 and 89 mg/dL; mean insulin levels were 40 and 3 μU/mL; and mean glucagon levels were 633 and 1150 pg/mL. The hepatic glycogen content of the animals, which had received intravenous dextrose, did not change significantly during the 8 Hs of hypothermic storage in Collins solution. A slow, but non significant decrease in glycogen content was observed in the livers from the fasted rats.13
Studies performed with pigs demonstrated that intraportal glucose infusion produces rapid and substantial hepatic glycogenation. Taking into account these findings, glycogen content and degradation in human livers were determinate during transplantation. Moreover, the effect of intraportal glucose-insulin infusions during the donor operation on these variables was also studied, finding that intraoperative glucose infusions in humans can reglycogenate the liver and increase glycogen degradation.31
Livers from fasted donors appear to tolerate longterm preservation better than livers from fed donors.32 Lindell et al., obtained 31% survival after 40-Hour preservation of livers from fed donor rats and 67% survivors with donor livers from 4-day fasted rats. The explanation for this improvement is not known but may be due to inactivation of Kupffer cells due to nutritional depletion of the liver. Kupffer cell activation has been one hypothesis to explain how cold storage injuries livers during reperfusion (transplantation). Although livers from fasted donors showed improved survival, there was extensive hepatocellular injury as indicated by large LDH release from the livers after 40-Hour cold storage as tested by isolated perfusion. AST release showed a similar pattern and bile production was more suppressed in livers from fasted donors than fed ones. This dilemma (both fasting and feeding improved survival) is discussed in terms of how the interactions between Kupffer cells and hepatocytes affect liver viability. Donor fasting is probably impractical clinically as a method to improve the donor liver, but elevating liver glycogen by glucose supplementation is possible and may lead to improved preservation and outcome in liver transplantation.33
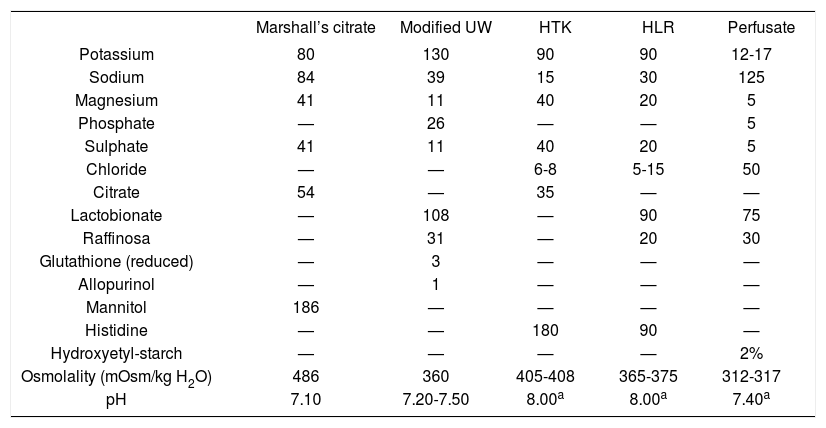
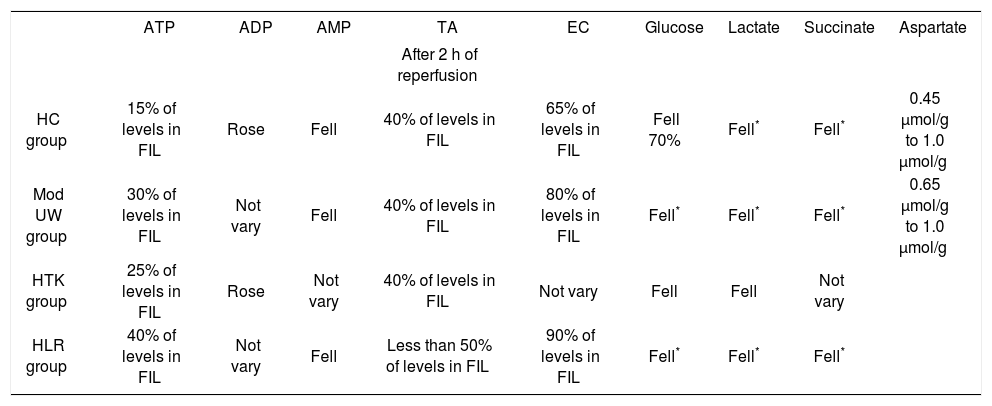
Restoration of hepatic energy metabolism using different cold preservation solutionsMitchell et al, compared four different preservation solution to study the metabolic damage caused during the cold ischemic period. The solutions were: modified UW solution; hypertonic citrate solution (HC–Marshall’s citrate); Bretschneider’s histidine-based solution (HTK) and HLR solution (histidine-lactobionate-raffinose, which is the combination of the impermeant anion from UW solution latobionate with the histidine buffer from HTK solution).11
Non-fasting male rats (200–300 g) were used and anaerobic glycolisis was studied during cold reperfusion at 4ºC. Livers were flushed with different preservation solutions and then stored in 50 ml of the flush solution on ice during 24 Hs. Then, the organs were connected to a reperfusion circuit and reperfused for 2 Hs with an airequilibrated, recirculating system passing through a 2 μm filter. The whole apparatus was kept in a thermostatically controlled environment at 4ºC. The same perfusate was used for all groups (it was a solution with a similar electrolyte composition, osmolality, an pH to plasma). Table III shows the composition of the preservation solutions and perfusate.11ATP (adenosine triphosphate), ADP (adenosine diphosphate), AMP (adenosine monophosphate), TA (adenine nucleotide levels), EC (energy charge), glucose, lactate, succinate and aspartate were measured. Table IV shows the results for all groups.
Composition of the preservation solutions and perfusate (mmol/liter):a The pH was adjusted with HCl (histidine-based and the HLR solutions) and with KOH (perfusate) at room temperature.
| Marshall’s citrate | Modified UW | HTK | HLR | Perfusate | |
|---|---|---|---|---|---|
| Potassium | 80 | 130 | 90 | 90 | 12-17 |
| Sodium | 84 | 39 | 15 | 30 | 125 |
| Magnesium | 41 | 11 | 40 | 20 | 5 |
| Phosphate | — | 26 | — | — | 5 |
| Sulphate | 41 | 11 | 40 | 20 | 5 |
| Chloride | — | — | 6-8 | 5-15 | 50 |
| Citrate | 54 | — | 35 | — | — |
| Lactobionate | — | 108 | — | 90 | 75 |
| Raffinosa | — | 31 | — | 20 | 30 |
| Glutathione (reduced) | — | 3 | — | — | — |
| Allopurinol | — | 1 | — | — | — |
| Mannitol | 186 | — | — | — | — |
| Histidine | — | — | 180 | 90 | — |
| Hydroxyetyl-starch | — | — | — | — | 2% |
| Osmolality (mOsm/kg H2O) | 486 | 360 | 405-408 | 365-375 | 312-317 |
| pH | 7.10 | 7.20-7.50 | 8.00a | 8.00a | 7.40a |
Extracted from reference 11.
Comparison of different parameters measured for each preservation solution: FIL: freshly isolated rat liver;* significantly.
| ATP | ADP | AMP | TA | EC | Glucose | Lactate | Succinate | Aspartate | |
|---|---|---|---|---|---|---|---|---|---|
| After 2 h of reperfusion | |||||||||
| HC group | 15% of levels in FIL | Rose | Fell | 40% of levels in FIL | 65% of levels in FIL | Fell 70% | Fell* | Fell* | 0.45 μmol/g to 1.0 μmol/g |
| Mod UW group | 30% of levels in FIL | Not vary | Fell | 40% of levels in FIL | 80% of levels in FIL | Fell* | Fell* | Fell* | 0.65 μmol/g to 1.0 μmol/g |
| HTK group | 25% of levels in FIL | Rose | Not vary | 40% of levels in FIL | Not vary | Fell | Fell | Not vary | |
| HLR group | 40% of levels in FIL | Not vary | Fell | Less than 50% of levels in FIL | 90% of levels in FIL | Fell* | Fell* | Fell* |
Based on Mitchell et al.
This study demonstrates that hypothermic reperfusion is capable of restoring hepatic energy metabolism after prolonged cold ischemic storage. Even after a considerable cold ischemia time of 24 Hs, ATP and EC were restored, two key parameters of cellular energetic. Storage in HLR solution facilitated the resuscitation of energetic. Modified UW and HLR stored livers had preserved TA significantly greater than Marshall’s citrate or HTK.
The benefit of using HLR is that this solution has the osmotic, impermeant effects of the lactobionate and raffinose, reducing hypothermia-induced cell swelling, combined with the added buffering capabilities of histidine.11
Insulin and University of Wisconsin solutionUW solution proved to be able to prevent liver injury during cold ischemia. UW solution has basic components, which cannot be omitted, and others that are additives and could be replaced or omitted. Lactobionate, raffinose and glutathione are the basic components, which must be, present in UW solution to warrantee its efficacy. One of the additives components is insulin, whose role in the solution is still controversial.34 Insulin should be used as component of UW solution because is well known that promotes anabolic growth and to co-stimulate liver regeneration. It is considered a nutritional factor for liver and is widely used in liver transplantation. Insulin was added to UW solution with the aim of stimulating glycolysis during preservation. However, during the storage period of the liver graft, insulin may actually render the preserved liver graft active and, in the absence of glucose in UW solution, may deplete energy storage of the liver cells.35
Yu et al., found that the survival rate of the rats receiving liver graft stored in UW with insulin was much lower than that of the group without insulin after 8 Hs of preservation.36 Insulin keeps the liver in a metabolically vigorous state. However, organ preservation aims to decrease the metabolic rate.35
Preserved livers in UW solution with insulin increased transaminases levels and mortality. Moreover, the expressions of 215 genes were repressed, such as fatty acid-binding protein genes, insulin-like growth factor-binding protein genes, apolipoprotein D precursor genes, among others, after 24 Hs of cold preservation.35
It is clear that insulin in UW solution can exacerbate ischemic injury and decrease the graft survival rate in rat liver transplantation.
In summary there is still controversy about what is the best donor nutritional status and its influence on glycogen preservation. Perhaps, there might not be only one condition that provides protection against preservation/reperfusion injuries and the possibility of restoring energy storage properly during reperfusion at the same time. The combination of the best preservation solution to facilitated resuscitation of energetic and liver supplementation with drugs or metabolites may be the clue for good glycogen storage preservation during cold schema/reperfusion period.