The prevalence and incidence of alcoholic liver disease are constantly evolving. Alcoholic liver disease has a wide clinical spectrum. It may progress to cirrhosis and to end-stage liver disease requiring liver transplantation. The histological manifestations range from steatosis without inflammation to liver cell injury and ultimately to fibrosis and cirrhosis. In some cases, the histological manifestation is steatohepatitis, morphologically characterized by inflammation and necrosis. Currently, although there are no specific tests to establish a diagnosis of steatohepatitis, some serological, radiological, or laboratory tests may be useful. Liver biopsy is useful in confirming a suspected diagnosis and in assessing the extent of parenchymal damage. This review synthesizes the main aspects of the epidemiology, pathogenesis, morphological characteristics, diagnosis, treatment, and prognosis of alcoholic liver disease.
Cirrhosis causes nearly 150,000 deaths each year worldwide, and alcoholic cirrhosis accounts for approximately 38–50% of all cirrhosis-related deaths (Figure 1).1,2 Alcohol liver disease (ALD) occurs in patients who consume excessive amounts of alcohol. Approximately 7.4% of the U.S. population meets the diagnostic criteria for alcohol abuse or alcoholism; in Europe, 20–30% of the population consumes excessive amounts of alcohol; and in many developing nations, the increase in alcohol consumption is alarming. In Mexico, cirrhosis is the third leading cause of death in adults between 45 and 60 years of age, and alcohol is related to more than 50% of these deaths. It is estimated that by the year 2050, there will be more than one million cases of ALD in Mexico.3-10
Usually, alcohol-related problems are not detected until a decompensated state occurs.11 Up to 90% of alcoholics have fatty liver, and over a 20-year follow-up period, 5–15% of these patients will develop cirrhosis.12,13 In 1995, Teli et al. published data demonstrating that patients with fatty liver can progress to cirrhosis. In that study, patients with alcoholic fatty liver had a 10% risk of progressing to cirrhosis over a period of 10.5 years and an 18% risk of cirrhosis or fibrosis. The risk of developing cirrhosis increased to 30% and the risk of developing of cirrhosis or fibrosis to 37% in those who continued to drink alcohol.14 About 10–35% of alcoholics exhibit changes on liver biopsy consistent with alcoholic hepatitis.15 The probability of developing cirrhosis in this group of patients is approximately 10–20% per year, and approximately 70% will finally develop cirrhosis. In 10% of patients, the changes associated with alcoholic hepatitis can be reversed and the liver function normalized with complete cessation of alcohol intake.16
Alcohol abuse and alcoholic liver disease are found predominately in men. However, 13–33% of Americans who either abuse or depend on alcohol are women. Alcohol consumption by women is increasing in the United States, as well as in Europe and Asia. Women consume less alcohol on average than men and are less likely to be heavy users, but the duration of drinking is similar in both sexes.17,18 Drinking often starts at a relatively young age. The age group at highest risk for hospitalization due to alcohol- related liver disease is between 45 and 64 years, with a prevalence of 94.8 per 10,000. The overall prevalence decreases with increasing age, but the prevalence remains higher for men than for women.19
The death rate among blacks with ALD exceeds that of whites. Stinson et al. analyzed alcoholic cirrhosis mortality from 1991 to 1997, and the rank order of mortality rates for men, from highest to lowest, was white Hispanic, black non-Hispanic, white non-Hispanic, and black Hispanic.20 It is not clear whether ethnic differences in the rates of alcoholic cirrhosis and alcoholic liver disease are due to genetic factors or to differences in the amounts and types of alcohol consumed, or whether they are related to differences in socioeconomic status and access to medical care.21
It is estimated that the prevalence of hepatitis C in patients with alcoholic liver disease ranges from 14% to 43%. Both alcohol (daily alcohol intake > 50 g/d) and hepatitis C are independent risk factors for the development of cirrhosis. Alcoholics with hepatitis C infection have higher levels of virus, are more likely to develop fibrosis and cirrhosis, and develop cirrhosis more rapidly than do alcoholic patients without hepatitis C.22,23 The interaction of alcohol and hepatitis B is incompletely understood.
Nutrition and ALDMalnutrition is not an important risk factor for the development of ALD. However, there are some nutritional factors that promote liver disease. Alcohol provides 7.1 kcal/g and constitutes 5% of all caloric energy in the American diet. Malnutrition is always present to varying degrees when chronic alcoholism progresses to ALD.24 Alcohol is metabolized rapidly in the liver with no net energy storage. The substitution of alcohol for normal calories results in weight loss, as seen in chronic addicted alcoholics. The effects of alcohol consumption on diminishing fat oxidation can contribute to weight gain in a setting in which large amounts of alcohol are combined with a typical highfat diet.25 Weight loss in ALD is caused by three factors: anorexia with decreased intake of energy and protein-rich food, intestinal maldigestion of fat and protein, and a catabolic state that promotes gluconeogenesis from endogenous skeletal and visceral proteins.
Among alcoholics, folate deficiency is manifest clinically as macrocytic anemia and by morphological changes in enterocytes, and it may contribute to elevated levels of circulating homocysteine. The causes of folate deficiency include dietary deficiency, intestinal malabsorption, reduced liver uptake, increased renal loss, and reduced liver storage of folate.26 Thiamine deficiency is common in chronic alcoholics and is clinically apparent as Wernicke– Korsakoff disease with ophthalmoplegia, polyneuropathy, loss of short-term memory and cognition, confusion, and disordered gait. The causes of thiamine deficiency in alcoholism include poor diet and reduced intestinal absorption.27 Pyridoxine or vitamin B6 deficiency is expressed clinically as peripheral neuropathy, sideroblastic anemia, and a disproportionate ratio of aspartate transaminase (AST) to alanine transaminase (ALT). Pyridoxine deficiency in alcoholics is related to ethanol metabolism in the liver because the production of acetaldehyde results in the displacement of pyridoxal phosphate from albumin, followed by urinary excretion of the unbound vitamin. Serum vitamin A is usually maintained at normal levels in chronic alcoholics, but vitamin A levels are universally depleted in liver biopsies from ALD patients. This may be due to maldigestion secondary to a decrease in pancreatic esterase and reduced micelle incorporation.28
PathogenesisThe enzymes involved in the transformation of ethanol to acetaldehyde are gastric alcohol dehydrogenase (ADH), hepatic ADH, and the microsomal oxidative system, particularly cytochrome P-450 2E1 (CYP2E1), after which acetaldehyde is transformed to acetate via the acetaldehyde dehydrogenase pathway.8 The expression and activity of CYP2E are induced after ethanol consumption, indicating their role in the progression of ALD.
The development of ALD is influenced by nutritional factors, female sex, hepatitis viral infection, genetic predisposition, age, and intake of other drugs that induce CYP2E1. The nature of ALD is multifactorial, with complex interactions. The primary factors involved in the development of ALD are acetaldehyde, oxidative stress, hypoxia, membrane changes, and the immune response (Figure 2)29
The liver is composed of hepatocytes and nonparenchymal liver cell (endothelial cells, Kupffer cells/hepatic macrophages, hepatic stellate cells, bile duct epithelial cells, and pit cells/liver NK cells). Hepatocytes are the site of ethanol oxidation and ethanol-induced injury. Nonparenchymal liver cells represent only one-third of total liver cells and have important cellular functions, supporting liver homeostasis and actively participating in pathological processes. For example, Kupffer cells have a direct regulatory role in hepatocyte injury caused by ethanol by expressing tumor necrosis factor (TNF)-α.
ALD affects many of the enzymatic steps in methionine metabolism. The first step is the formation of S-adenosylmethionine (SAM), catalyzed by methionine adenosyltransferase (MAT). Levels of methionine are elevated in patients with alcoholic cirrhosis, which is attributed to a 50–60% decrease in MAT activity. This reduction in MAT activity may be the result of a change in the equilibrium of the liver-specific MAT isoform or covalent modification of the enzyme. The decline in MAT activity results in depletion of hepatic SAM and glutathione (GSH), and decreased transmethylation. Important consequences include the impairment of antioxidant defenses and changes in phospholipid composition, membrane fluidity, gene expression, and DNA stability. Impaired methionine resynthesis contributes to liver injury by decreasing the availability of SAM, with the consequences already described.
Changes in homocysteine metabolism result in increased homocysteine released by hepatocytes, which contributes to fibrogenesis. Mitochondria produce more reactive oxygen species (ROS) with ethanol consumption, a defect that is caused by impaired transport of GSH into the mitochondria. Reduced SAM levels can also affect mitochondrial GSH transport, and cells are sensitized to oxidative- stress-induced injury by this mechanism.30 Chronic ethanol consumption causes decreases in cellular ATP content and mitochondrial membrane potential. Ethanol also causes modifications to, and single-stranded breaks in, mitochondrial DNA. What determines the type of cell death (apoptosis or necrosis) in ALD is currently unknown, but the extent of ATP depletion may be critical.31
Another pathogenic mechanism relates to nuclear factor kappa-beta (NF-kβ). In experimental animal models of ALD, there is increased expression of NF-kβ, with inhibition of DNA synthesis and impaired liver regeneration.
Recent studies have shown that leptin, which normally regulates appetite, metabolic rate, and fat deposition, is increased in ALD and may have a role modulating the phenotype of hepatic macrophages.32 Ethanol induces changes in sinusoidal endothelial cells, causing reduced fenestration and hyaluronan uptake and impairment of receptormediated endocytosis. Intracellular adhesion molecule 1 (ICAM-1) expression by endothelial cells is up-regulated in experimental ALD and correlates with plasma endotoxin, hepatic TNF-α mRNA, and liver inflammation and injury.33,34
Hepatic stellate cells are involved in liver homeostasis including vitamin A storage, regulation of sinusoidal blood flow, communication between hepatocytes, and the maintenance of the hepatocyte phenotype. They also regulate matrix remodeling and the regulation of local inflammation. Oxidative stress is particularly relevant to the mechanisms of hepatic stellate cell stimulation in alcoholic liver fibrogenesis. Collagen synthesis is induced by 4-hydroxynonenal (a lipid peroxidation product), and oxidative stress may contribute to the activation of hepatic stellate cells. Hyperhomocystenemia may also contribute to the activation of hepatic stellate cells. Increased homocysteine released by hepatocytes in ethanol-induced liver injury due to abnormal methionine metabolism can exert paracrine effects on stellate cells to induce fibrogenesis.35
Alcoholism is believed to result from interactions between genetic predisposition and environmental factors. The genes encoding the enzymes involved in alcohol metabolism (alcohol dehydrogenase and aldehyde dehydrogenase) display functional polymorphisms. Because ADH2*2, ADH3*1, and ALDH2*2 are thought to protect individuals from developing alcoholism, these polymorphisms should also protect them from developing ALD.36
PathologyThere are three main forms of ALD (see Table I). The lesions and microscopic findings of ALD are variable, and they include liver steatosis, ballooning degeneration, apoptosis, Mallory’s hyaline, megamitochondria, neutrophilic infiltrates, lipogranulomas in the lobules, perisinusoidal collagen, perivenular fibrosis or veno-occlusive lesions, lymphocytic or neutrophilic inflammation, bile ductular proliferation, and periportal fibrosis.37
Liver cell ballooning, Mallory’s hyaline, and perivenular and perisinusoidal fibrosis predominate in acinar zone 3. In alcoholic hepatitis, the zone 3 group of lesions is known as “sclerosing hyaline necrosis”. With progression, cirrhosis may occur with iron accumulation in the hepatocytes and Kupffer cells.38,39
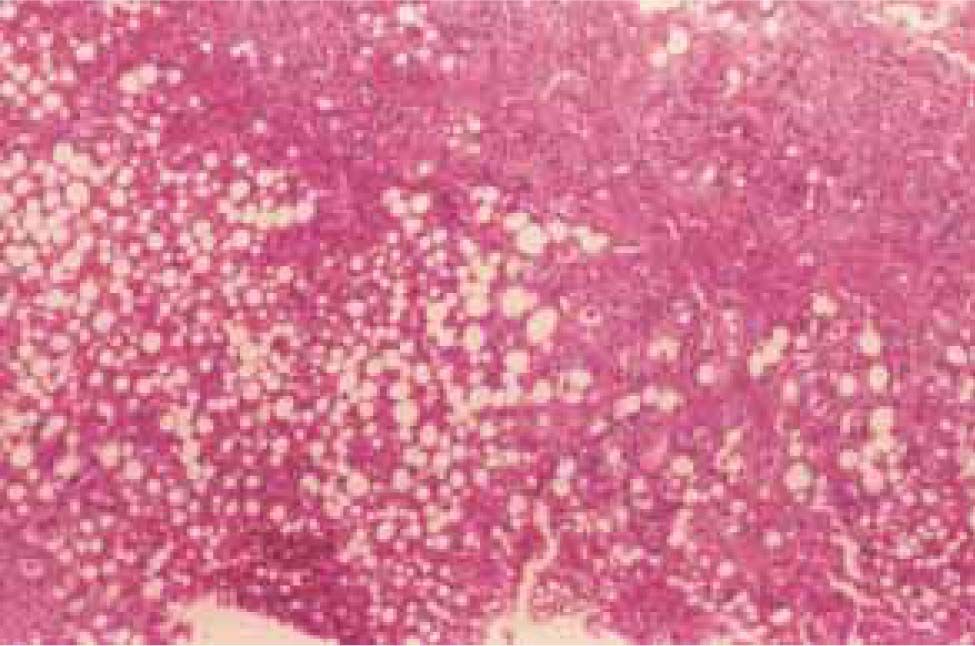
Steatosis, described as macrovesicular, microvesicular, or mixed, is the earliest histopathological manifestation of ALD. Macrosteatosis, more common in alcoholic steatohepatitis, is characterized by single large vacuoles within hepatocytes that displace the cytoplasm and nucleus. It occurs as a result of metabolic imbalances in lipid delivery or synthesis within the liver, intracellular oxidation, and export from hepatocytes resulting in triglyceride accumulation. A mild degree of macrovesicular steatosis is a common finding in most liver biopsies (Figure 3)40,41
Lipogranulomas are comprised of single or multiple fat globules surrounded by chronic inflammatory cells and Kupffer cells, sometimes with a few eosinophils.42 The presence of Mallory’s hyaline in noncirrhotic zone 3 liver cells is characteristic of ALD. The presence of megamitochondria in a liver with fat may indicate chronic alcohol abuse. They are observed by light microscopy as round or cigar-shaped (Figure 4)43
For a diagnosis of steatohepatitis, features of liver cell injury should be identified in a liver biopsy. The most common form of injury is ballooning degeneration, characterized by swollen hepatocytes with rarefied cytoplasm, typically found in zone 3. Other common findings include septa of bridging necrosis and acidophil bodies, which are histological markers of apoptotic necrosis. There may be lobular infiltrates of polymorphonuclear leukocytes within the sinusoids and adjacent to injured liver cells. These are considered to be an immune-mediated feature causing direct cytopathic damage to hepatocytes by the release of neutrophil lysosomal granules (Figure 5) Mononuclear cell inflammation of a mild degree may also be noted and sometimes represents a resolution. Sometimes there is a deposition of collagen in the space of Disse (perisinusoidal fibrosis), initially located in acinar zone 3; with progression to fibrosis, septum formation, and linkage between the central and portal regions, eventual cirrhosis may occur (micronodular, macronodular, or mixed). The presence of activated hepatic stellate cells in alcoholic steatohepatitis has been documented. Lesions of the hepatic veins are characteristic features of ALD. These include thickened veins and perisinusoidal fibrosis.44
The revised hepatotoxic threshold in which ALD develops is 40 g (four drinks) and 20 g (two drinks) daily for men and women, respectively.14,45
All physicians should ask their patients about alcohol use. Standard validated questionnaires are more effective in the detection and diagnosis of ALD than routine clinical or laboratory tests. The usual questionnaire is the CAGE questionnaire (Table II). A positive answer for two or more questions indicates a positive result. A more recent test is the Alcohol Use Disorders Identification Test (AUDIT) published by the World Health Organization (Table III). Patients who admit to drinking over the toxic threshold or who have positive responses to questionnaires should undergo a more complete evaluation for alcohol use and dependence.46 According to the American Psychiatric Association’s Diagnostic and Statistical Manual of Mental Disorders (4th edition) (DSM-IV), at least one of the following criteria is required for a diagnosis of alcohol abuse: failure to fulfill social obligations; recurrent substance use in physically hazardous situations; recurrent legal problems; and continued use despite alcohol- related interpersonal or social problems. For alcohol dependence, at least three of the following criteria must be present: tolerance; withdrawal symptoms; use of alcohol in larger quantities than intended; continued desire to control use or cut down; significant time lost because of obtaining, using, or recovering from alcohol; sacrifice of social, occupational, or recreational tasks; and continued use despite physical and psychological problems. These conditions should alert clinicians to look for clinical and laboratory evidence of ALD.47
CAGE Questionnaire.
| •Have you ever felt the need to cut down on drinking? |
| •Have you ever felt annoyed by criticism of your drinking? |
| •Have you ever felt guilty about your drinking? |
| •Have you ever taken a morning eye opener? |
Interpretation: Two “yes” answers are considered a positive screen. One “yes” answer should arouse a suspicion of alcohol abuse.
Interpretation: Modified from Mayfield D et al. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131:1121.
The alcohol use disorder identification TEST (AUDIT).
| Scores for response categories are given in parentheses. Scores range from 0 to 40, with a cutoff score of > 5 indicating hazardous drinking, harmful drinking, or alcohol dependence. |
| 1.How often do you have drinking containing alcohol? |
| (0)Never |
| (1)Monthly or less |
| (2)Two to four times a month |
| (3)Two or three times a week |
| (4)Four or more times a week |
| 2.How many drinks containing alcohol do you have on a typical day when you are drinking? |
| (0)1 or 2 |
| (1)3 or 4 |
| (2)5 or 6 |
| (3)7 to 9 |
| (4)10 or more |
| 3.How often do you have six or more drinks on one occasion? |
| (0)Never |
| (1)Less than monthly |
| (2)Monthly |
| (3)Weekly |
| (4)Daily or almost daily |
| 4.How often during the past year have you found that you were not able to stop drinking one you had started? |
| (0)Never |
| (1)Less than monthly |
| (2)Monthly |
| (3)Weekly |
| (4)Daily or almost daily |
| 5.How often during the past year have you failed to do what was normally expected of you because of drinking? |
| (0)Never |
| (1)Less than monthly |
| (2)Monthly |
| (3)Weekly |
| (4)Daily or almost daily |
| 6.How often during the past year have you needed a first drink in the morning to get yourself going after a heavy drinking session? |
| (0)Never |
| (1)Less than monthly |
| (2)Monthly |
| (3)Weekly |
| (4)Daily or almost daily |
| 7.How often during the past year have you had a feeling of guilt or remorse after drinking? |
| (0)Never |
| (1)Less than monthly |
| (2)Monthly |
| (3)Weekly |
| (4)Daily or almost daily |
| 8.How often during the past year have you been unable to remember what happened the night before because you had been drinking? |
| (0)Never |
| (1)Less than monthly |
| (2)Monthly |
| (3)Weekly |
| (4)Daily or almost daily |
| 9.Have you or has someone else been injured as a result of your drinking? |
| (0)No |
| (1)Yes, but not in the past year (4) Yes, during the past year |
| 10.Has a relative or friend or a doctor or other health worker been concerned about your drinking or suggested you cut down? |
| (0)No |
| (1)Yes, but not in the past year (4) Yes, during the past year |
From Piccinelli M et al: Efficacy of the alcohol use disorders identification test as a screening tool for hazardous alcohol intake and related disorders in primary care. A validity study. BMJ 1997;314:420.
The presentation of ALD correlates with the degree of liver injury. Alcoholic fatty liver has a milder clinical presentation than alcoholic hepatitis or cirrhosis. Fatty liver occurs after short-term binge drinking and is the hallmark of acute ingestion. Patients are usually asymptomatic and have normal to mildly abnormal liver tests. Once alcohol ingestion ceases, the steatosis typically resolves. In approximately 20% of patients, long-term alcohol consumption in those with alcoholic fatty liver may result in liver fibrosis and cirrhosis.13 Physical examination may reveal jaundice, parotid hypertrophy, spider angiomata, gynecomastia, palmar erythema, Dupuytren’s contracture, and testicular atrophy. Table IV shows some of the physical signs associated with cirrhosis.
Physical signs in cirrhosis.
| •Ascites |
| •Splenomegaly |
| •Spider angiomata |
| •Dupuytren’s contractures |
| •Palmar erythema |
| •Hipocratic fingers |
| •Periferic neuropathy |
| •Asterixis |
| •Gynecomastia |
| •Testicular atrophy |
Modified from Méndez-Sánchez N, Uribe M. Conceptos actuales en hepatología Masson Doyma México. 2003:259-264.
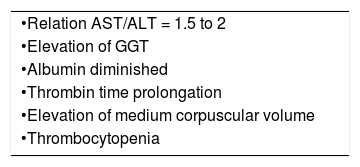
Patients with alcoholic hepatitis may be asymptomatic or may present with fever, jaundice, hepatomegaly, and occasionally ascites, portal hypertensive bleeding, and hepatic encephalopathy. On laboratory examination, leukocytosis is often present. A serum AST/ALT ratio greater than 2.0 helps to differentiate ALD from other liver diseases. Low ALT elevation is the result of a hepatic deficiency of pyridoxal-6-phosphate, necessary for ALT enzymatic activity. Approximately 70% of patients with alcoholic hepatitis have cirrhosis.48,49 The clinical and laboratory features (AST/ALT ratio, gamma glutamyl transferase [GGT], mean corpuscular volume [MCV]) are approximately 79% sensitive and 98% specific for the diagnosis of ALD.
Ultrasonography or computed tomography may be useful for the evaluation of cirrhosis and portal hypertension. On ultrasound, the features of cirrhosis and portal hypertension include liver nodules, sluggish or reversed portal vein flow, splenomegaly, and intraabdominal varices.
Patients with alcoholic cirrhosis have clinical features similar to patients with cirrhosis from other causes. Laboratory abnormalities are suggestive of liver synthetic dysfunction, such as coagulopathy, hypoalbuminemia, and hyperbilirubinemia. In decompensated patients, the presentation includes ascites, encephalopathy, and portal hypertensive bleeding.
In some cases, liver biopsy is needed prior to treatment to confirm the diagnosis of alcoholic hepatitis when alternative diagnoses are suspected. It is also useful before a decision is taken to treat with corticosteroids or other therapies, and it provides prognostic information on the severity of liver pathology.50 In patients with ALD and viral hepatitis, a liver biopsy is useful to determine the degree of necroinflammatory features, to make treatment decisions, and to obtain prognostic information: patients with ALD and hepatitis C have more significant liver disease, higher mortality, and a higher risk of hepatocellular carcinoma.51
Chronic alcohol consumption reduces the number of carbohydrate moieties attached to transferrin, leading to carbohydrate- deficient transferrin (CDT). Compared with other tests for alcohol use, the CDT test is more sensitive and specific for alcohol abuse. The limitations of CDT testing include wide ranges in sensitivity and specificity, and levels are affected by changes in serum transferrin or by hypertension, primary biliary cirrhosis, hepatic malignancy, chronic viral hepatitis, or decompensated liver disease.52 Compounds of alcohol metabolism, such as erythrocyte acetaldehyde and acetaldehyde adducts, have been shown to be effective markers for alcohol consumption but are not specific enough for clinical use.53
TreatmentAbstinence is the fundamental therapy for alcohol abuse. There is substantial evidence for the role of abstinence in the improvement of the end-organ effects of alcohol abuse in the heart, brain, pancreas, gastrointestinal tract, and reproductive organs. Continuing alcohol consumption is the best predictive factor of poor outcome. The diverse presentations of ALD (fatty liver, alcoholic hepatitis, and cirrhosis) are likely to improve or stabilize with the cessation of alcohol use and concurrent attention to malnutrition.54 Malnutrition in ALD patients should be corrected with a diet of protein equivalent to 25–35 kcal and 1.25 g per kilogram of ideal body weight.8 Studies have demonstrated that patients who consumed at least 2,500 kcal per day had improvements in six-month survival, liver function, and nutritional status compared with a group that had a lower voluntary intake of calories.55 Enteral nutrition feeding is safe, maximizes digestion, and may improve long-term survival in patients with ALD.56 An analysis of seven studies involving 239 ALD patients concluded that parenteral nutrition treatment for periods of up to 30 days may improve liver function and nitrogen balance while normalizing the composition of plasma amino acids, but the long-term metabolic effects, risks, and benefits remain uncertain.57
Amino acid formulas enriched in branched-chain amino acids (BCAA; leucine, valine, and isoleucine) appear to confer an advantage by competing with aromatic amino acids for blood–brain barrier transport, improving hepatic encephalopathy, and stimulating protein synthesis, which could benefit the resolution of ascites. To date, there have been no studies comparing the potential benefits of BCAA with standard amino acid formulations in parenteral or enteral formulas.58
Obesity is a risk factor for the progression to ALD. However, aggressive weight reduction may not help in the treatment of alcoholic fatty liver and can be associated with a worsening of liver injury.59
Treatment with S-adenosylmethionine (SAM) prevents the depletion of SAM and GSH levels and ameliorates liver injury, including fibrosis. The protective effects are mediated via increased GSH levels. The administration of SAM prevents GSH uptake into the mitochondria. SAM also reduces liver injury by inhibiting TNF-α release from macrophages, either by itself or via its nucleoside metabolite 5-methylthioadenosine (MTA).60,61 SAM also attenuates the production of inducible nitric oxide synthase and the activation of NF-kβ. It also induces the expression and production of cytoprotective interleukin-10 (IL-10). SAM can also reduce alcoholic liver injury by lowering levels of homocysteine, which may be implicated directly in hepatocellular apoptosis. Depletion of SAM may lead to a reduction in phosphatidylcholine. The administration of phosphatidylcholine has been advocated for the prevention and treatment of ALD because of its antifibrotic effects, which include a decrease in the transformation of intrahepatic lipocytes to collagen-producing stellate cells, the induction of collagenase activity, the suppression of platelet-derived growth factor (PDGF), induced proliferation of hepatic stellate cells, protection against oxidant stress, and suppression of CYP2E1 induction.62,63
Betaine also facilitates phosphatidylcholine generation and very low-density lipoprotein export from the liver, and it offers the additional benefit of decreasing serum homocysteine levels.64 Antioxidants exert beneficial effects on hepatocytes by desensitizing them to oxidant stress, while inhibiting the priming mechanism for the expression of proinflammatory and cytotoxic mediators via the suppression of NF-kβ.65 General measures recommended for patients with ALD include the management of insulin resistance and the treatment of alcohol withdrawal symptoms with benzodiazepines.
Patients with suspected alcoholic hepatitis with decompensated liver disease (encephalopathy, hyperbiliru-binemia above 5 mg/dL, and coagulopathy) have a poor prognosis, with mortality rates as high as 50% in one year. The use of corticosteroids in selected patients with severe alcoholic hepatitis has been supported. The usefulness of a Maddrey Discriminant Factor (MDF) score (4.6 × [prolongation of the prothrombin time in seconds] + total serum bilirubin in mg/dL) greater than 32 in predicting increased mortality in alcoholic hepatitis has been validated.66 A recent meta-analysis concluded that corticosteroids improved the short-term survival of patients with severe alcoholic hepatitis by approximately 20%. Corticosteroids are given as oral prednisolone (32 mg) or prednisone and methylprednisolone (40 mg) daily for four weeks, followed by a four-week taper. The improvement in survival, lack of serious immediate side effects or complications, and the low relative cost support the use of corticosteroids.67,68
Pentoxifylline (400 mg orally three times daily) is an inhibitor of TNF-α and other cytokines. It offers an important option for the protection of renal function and improvement in short-term survival in severe alcoholic hepatitis.69 Pilot studies have shown that neutralizing anti-TNF-α agents (infliximab and etanercept) potentially interfere with an important mechanistic pathway of ALD.
Excessive consumption of alcohol leads to the generation of oxidative cellular stress and injury from increased endotoxemia, cytokine release, and membrane-bound lipid peroxidation. There is a broad spectrum of orally administered agents with antioxidant properties. Small pilot studies using N-acetylcysteine or vitamin E have shown no effect of these antioxidants in the context of severe alcoholic hepatitis. The long-term use of antioxidants may have a greater role in the treatment of cirrhotic disease, with the potential to prevent a decompensated state and the development of hepatocellular carcinoma.29
The cellular physiology and blood flow of the hepatic acinus leads to a relatively oxygen-poor microenvironment within the pericentral hepatocytes (zone 3) and an area with greater sensitivity to drug-induced, ischemic, and alcohol-induced injury. A “hypermetabolic” state is implicated in the pathogenesis of zone 3 alcoholic injury. Suppression of thyroid activity with propylthiouracil (PTU) has been proposed as a treatment for alcoholic injury. A systematic review combining the results of six randomized clinical trials demonstrated that PTU had no significant effect on mortality or liver-related mortality relative to the effects of placebos. Accordingly, there is no evidence to support the use of PTU for ALD.70
Some recent reports have demonstrated the regression or loss of hepatic fibrosis. Recognition of the importance of hepatic stellate cells and Kupffer cells as keys to the progression and regression of liver scarring offers new opportunities for cirrhosis therapies.71 Recently, clarification of the roles of leptin and adiponectin in insulin resistance and in the progression to chronic liver disease make these hormones candidates for future trials in patients with ALD.
Orthotopic liver transplantation (OLT) is the only effective treatment for patients with terminal ALD. Currently, ALD is the second leading indication for transplantation in the United States.72 Any patient with decompensated liver disease without contraindications for transplantation can be listed for OLT. Current or recent substance abuse is a contraindication for OLT. Proteincalorie malnutrition is a common comorbidity in alcoholic patients and patients with end-stage liver disease, and it presents a significant risk of a poor outcome. Psychiatric diseases commonly coexist with alcohol dependence. Personality disorders, depression, anxiety, or psychoses require psychiatric involvement.73 Significant cardiomyopathy is another contraindication for OLT. Current recommendations suggest pre-OLT cardiovascular assessment for symptomatic high-risk individuals with risk factors for coronary artery disease.74 Drinking relapse after OLT is common. Short-term recidivism rates range from 10–15% and cumulative relapse rates of 30–50% have been reported. The best predictor of continued sobriety after transplantation is a documented period of abstinence of six months or longer. Patient and graft survival rates after transplantation are similar to those of non-ALD patients, with 66–82% five-year survival.75Table VI shows the actual and available treatments for ALD.
PrognosisAs mentioned above, evidence suggests that with the ingestion of more than 60–80 g/d of alcohol in men and more than 20 g/d in women, there is an increased risk of cirrhosis. However, Bellentani et al. showed that only 13.5% of patients with extremely high daily alcohol intake (120 g/d) developed alcohol-induced liver damage.2,18 Alcohol consumption is necessary but not sufficient for the development of fibrosis in alcoholic patients. Cirrhosis occurs in only 8–20% and alcoholic hepatitis in 6–30% of alcoholic patients. Older, overweight, female, hyperglycemic, or iron-overloaded patients exhibit the risk factors for progression to fibrosis and cirrhosis. Age and female sex are independent risk factors for the development of alcohol-induced cirrhosis.
The long-term prognosis for patients with cirrhosis improves with abstinence. The five-year survival in compensated cirrhosis patients who continue to drink is less than 70% compared with a survival as high as 90% if they abstain from further alcohol intake. In patients with decompensated cirrhosis, the five-year survival rate is 60% in abstinent individuals but drops to less than 30% in those who continue to drink. Overall five-year survival in cirrhosis patients who continue drinking is approximately 35%.76
The prognosis for alcoholic hepatitis is highly variable. Patients with severe alcoholic hepatitis may develop rapid liver failure and die, or may recover with abstinence. The long-term prognosis for patients with less se-vere liver disease is mainly dependent on the cessation or persistence of drinking, but abstinence from ethanol does not completely prevent the development of cirrhosis.
Women have an increased susceptibility to the detrimental effects of alcohol. They are considered to be at increased risk of cirrhosis with a daily alcohol intake greater than 20–60 g. Progression to cirrhosis in women who abstain may not necessarily be related to more severe or more extended liver damage. Some unrecognized factors related to sex might be responsible for the unfavorable course of alcoholic hepatitis in some women even if they stop drinking. Gastric ADH is less active in women than in men, and its activity seems to be depressed in both alcoholic women and men.7,8,77,78 Other mechanisms underlying the increased susceptibility to alcohol-related liver injury in women include differences in endotoxin levels and gut permeability, the effect of estrogens and androgens on endotoxin–alcohol-mediated liver injury, differences in alcohol elimination rates either because of variations in first-pass metabolism or enzymatic activity, and differences in the volumes of distribution and peak blood alcohol levels.79,80
The role of obesity as a risk factor in the development of alcoholic cirrhosis is unclear. Evidence indicates that in alcoholic patients, being overweight for at least 10 years correlates independently with the occurrence of cirrhosis. Obesity is associated with steatohepatitis, and alcohol intake may increase liver damage in obese individuals.81
In addition to the directly toxic effects of alcohol and genetically based differences in alcohol-metabolizing enzymes, there are other genetically and environmentally determined factors that may predispose individuals to the development of ALD: obesity, genetically determined polymorphisms in cytokines and their receptors, immune mechanisms, patterns of alcohol consumption, concurrent viral infections, and the use of drugs.82
The long-term survival of alcoholic patients is adversely affected by the lesions that characterize alcohol steatosis and steatohepatitis. Histological lesions premonitory for the progression to cirrhosis include widespread liver cell necrosis and pericellular fibrosis, the formation of fibrous septa, widespread hepatic venular obstruction, and diffuse Mallory’s hyaline. Some lesions are reversible with abstinence from alcohol. The risk of developing cirrhosis is increased when steatohepatitis is present. Steatohepatitis may be considered the rate-limiting step in the progression to cirrhosis, and mortality due to alcoholic cirrhosis is adversely affected by its presence.83
The MDF (= 4.6 × [prothrombin time (PT) in seconds – control PT)] + serum bilirubin in mg/dL) was introduced in 1978 as a tool for predicting the risk of mortality from alcoholic hepatitis. Patients with a MDF score of more than 32 have a mortality rate exceeding 50% and corticosteroid treatment is suggested for this group of patients.65 The model for end-stage liver disease (MELD) is a survival model based on three laboratory variables: serum creatinine (Cr), serum bilirubin, and the international normalized ratio (INR) using the following formula: MELD = 9.57 × loge(Cr mg/dL) + 3.78 × loge(bilirubin mg/dL) + 11.20 × loge(INR) + 6.43.84 In a study undertaken to compare the prognostic validity of MELD and MDF in alcoholic hepatitis, both were similarly valid for 30-day and 90-day mortality, but MELD emerged as the only independent predictor of 90-day mortality. That study identified a MELD score of 21 as having the highest sensitivity and specificity in predicting mortality, with an estimated 90-day mortality of 20% for patients with this score.85
Physical examination for signs of encephalopathy or ascites may be useful in screening for mortality in alcoholic hepatitis. Alcohol can be considered both a primary cause of and a cofactor in the development of hepatocellular carcinoma (HCC). The risk of HCC increases when ethanol intake exceeds 60 g/d for more than 10 years. The rate of HCC increases significantly in patients with alcohol use and hepatitis C. Alcohol and hepatitis C interact in the development of HCC.86