Introduction and aim. We developed a rat model of portal vein ligation (PVL) with venous congestion (PVL+C) to investigate beneficial effect PVL plus congestion for regeneration of intact liver segments.
Materials and methods. In the PVL group, portal vein branches were ligated except the caudate lobe (CL). In the PVL + C group, the left lateral hepatic vein was ligated in addition to PVL. Chronological changes in the following variables were compared among the groups: CL weight to body weight ratio (CL/BW), embolized liver weight to body weight ratio (EL/BW), histological findings of the embolized/non-embolized liver, and expression of several mediators that affect liver regeneration in the non-embolized liver.
Results. Weight regeneration of CL continued up to postoperative day (POD)7 in PVL + C, but terminated at POD2 in PVL. CL/BW at POD7 was significantly higher in PVL + C than in PVL (2.41 ± 0.33% vs. 1.22 ± 0.18%, P < 0.01). In contrast, EL/BW continued to decrease up to POD7 in PVL + C but reached nadir at POD2 in PVL. Furthermore, EL/BW at POD7 was significantly smaller in PVL + C than in PVL (0.35 ± 0.03% vs. 0.67 ± 0.08%, P < 0.01). Histologically-proven injury in the embolized liver was more severe in PVL + C than in PVL. Expression of Ki-67, IL-6, TNF -a, and HGF were greater and/or more prolonged in PVL + C than in PVL.
Conclusions. Our rat model of PVL + C was considered useful for investigating the beneficial effect of congestion in addition to PVC. PVL + C caused increased devastation of the embolized liver, and higher and more prolonged expression of factors promoting liver regeneration in the non-embolized liver than in PVL.
Despite the introduction of highly effective non-surgical treatments for malignant tumors, only a hepatectomy can currently provide the chance of a cure for patients with hepatobiliary malignancy.1-4 Portal vein embolization or ligation (PVE/L) has been widely used for securing the safety of a subsequent major hepatectomy as well as expanding hepatectomy indications for patients with livers severely affected by hepatobiliary malignancy due to its ability to regenerate non-embolized liver, i.e. future liver remnant (FLR). However, 14-37% of patients receiving PVE/L have abandoned surgery due to insufficient regeneration of the FLR as well as oncological deterioration during the interval between PVE/L and hepatectomy.5-7
To compensate the above-stated concerns regarding PVE/L, i.e. insufficient FLR regeneration and modest regeneration velocity requiring 3-4 weeks for sufficient regeneration, hepatic venous embolization combined with PVE/L and associating liver partition and portal ligation for staged hepatectomy (ALPPS) were introduced and have been applied worldwide.8,9 In these two novel approaches, congestion in addition to PVE/L has been considered to have a central role in enabling a beneficial effect for regeneration of the non-embolized liver in terms of resultant FLR hypertrophy and/or earlier expansion of FLR.10,11 A clinical study by Hwan, et al. reported that congestion due to hepatic venous embolization performed subsequently to PVE/L caused greater FLR expansion than PVE/L alone.12 Furthermore, the earliness of FLR expansion and/or greater resultant of FLR brought on by ALPPS compared to PVE/L alone can be explained partly by partial congestion of the planned resected liver segments caused by venous disruption due to liver partition.10
Several animal models of PVE/L have been introduced to investigate the detailed mechanisms of the beneficial effect of PVE/L for regeneration of the non-embolized liver.13,14 However, to the best of our knowledge, an animal model of PVE/L with venous congestion to investigate the beneficial effect of congestion in addition to PVE/L has not yet been reported. In the present study, we developed a rat model of PVL with venous congestion (PVL + C) to determine the beneficial effect of congestion in addition to PVE/L in regeneration of the non-embolized liver in comparison PVL alone.
Materials and MethodsAnimalsEight-week-old male Wistar rats (Kureo, Tokyo, Japan) were housed in a room with a 12-h light/dark cycle and given full access to tap water and laboratory food. During all experimental procedures, animals were treated in accordance with the guidelines outlined in the Guide for the Care and Use of Laboratory Animals that was prepared by the US National Academy of Sciences and published by the National Institutes of Health (Bethesda, MD). The experimental protocol was approved by our institutional animal care committee (protocol number: F-A-14-008).
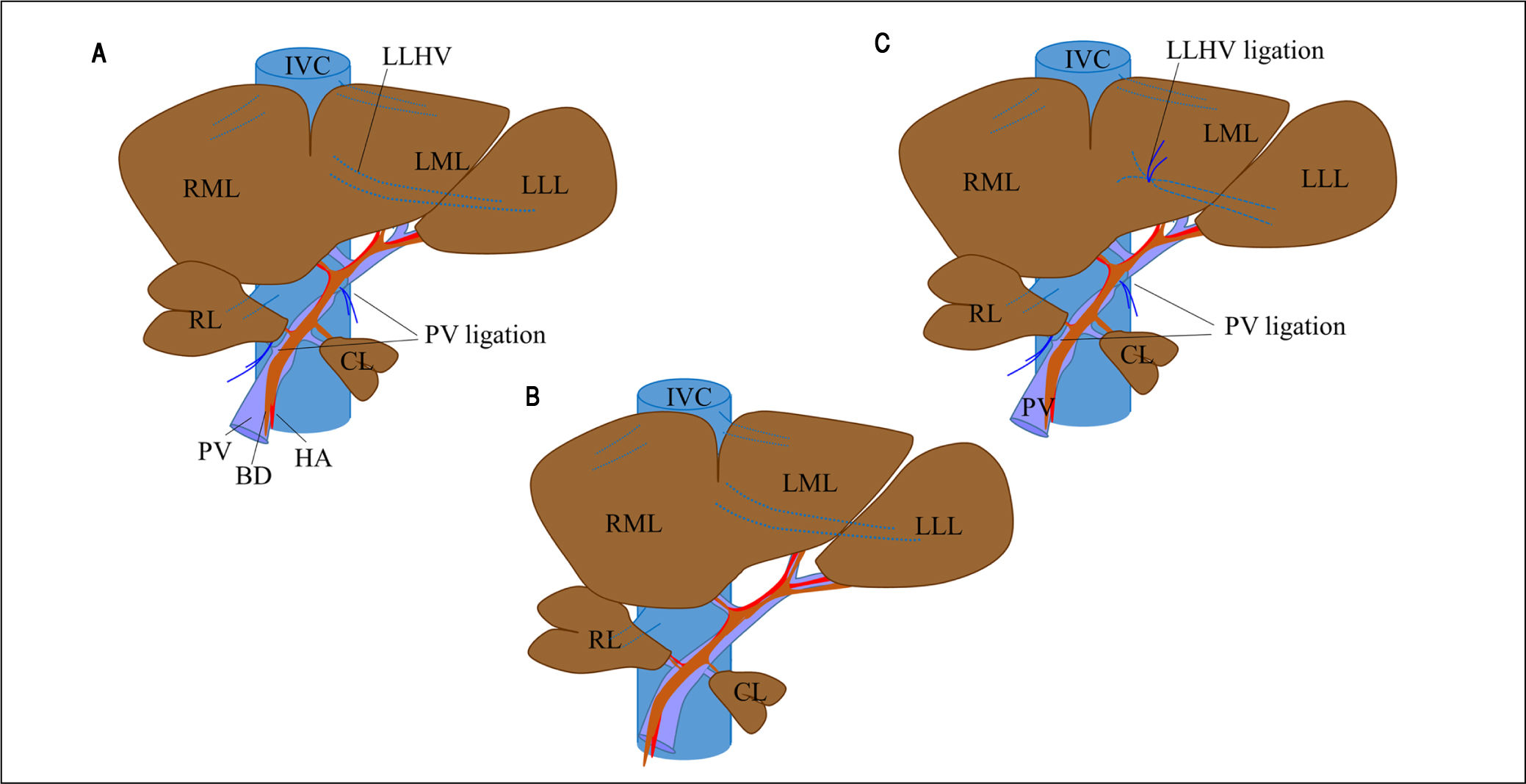
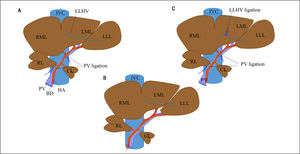
Experimental operative proceduresThe rat liver consists of four lobes (Figure 1C): the right lobe (RL); the middle lobe, which includes the right middle lobe (RML) and the left middle lobe (LML); the left lateral lobe (LLL); and the caudate lobe (CL). Each lobe is supplied by its own portal pedicle and is drained by its own hepatic veins.
Surgical procedure for PVL and in PVL with venous congestion (PVL + C) rat models. For PVL (A), PVL is performed for the right and left middle lobes, left lateral lobe, and right lobe. For PVL + C (B), the same PVL procedure is performed followed by induction of venous congestion by ligating the left lateral hepatic vein. The preoperative rat liver anatomy is shown in panel C (sham operation). PVL: portal vein ligation. CL: caudate lobe. RML: right middle lobe. LML: left middle lobe. LLL: left lateral lobe. PV: portal vein. IVC: inferior vena cava. HA: hepatic artery. LLHV: left lateral hepatic vein.
Rats were assigned at random to one of three experimental groups: PVL, PVL + C, and sham operation (SHAM). All surgical procedures were performed under anesthesia with isoflurane (concentration, 1.5%) and oxygen (flow rate, 0.5 L/min). In the PVL group (Figure 1A), selective PVL was performed to affect the RL, RML, LML, and LLL; after careful dissection sparing the hepatic arteries and bile ducts, portal veins corresponding to the lobes were ligated with 6-0 silk sutures. The CL was preserved and allowed to regenerate. In the PVL + C group (Figure 1B), PVL was performed as described above. Venous congestion then was induced by ligation of the left lateral hepatic vein. Special attention was paid to secure the integrity of the portal branches feeding the CL via the PVL and not to cause stenosis of the inferior vena cava and/or other hepatic veins via the lateral hepatic vein ligation. In the SHAM group (Figure 1C), the hepatic artery, PV, and bile duct were dissected without ligation, after which the abdomen was closed using double running sutures.
Arterial circulation and biliary duct branches were maintained in all rats. In both procedures including PVL, preservation of arterial flow reduced likelihood of the liver parenchyma becoming totally ischemic with subsequent abscess formation. No deaths or serious complications occurred during the surgical procedures or subsequent 7-d observation period.
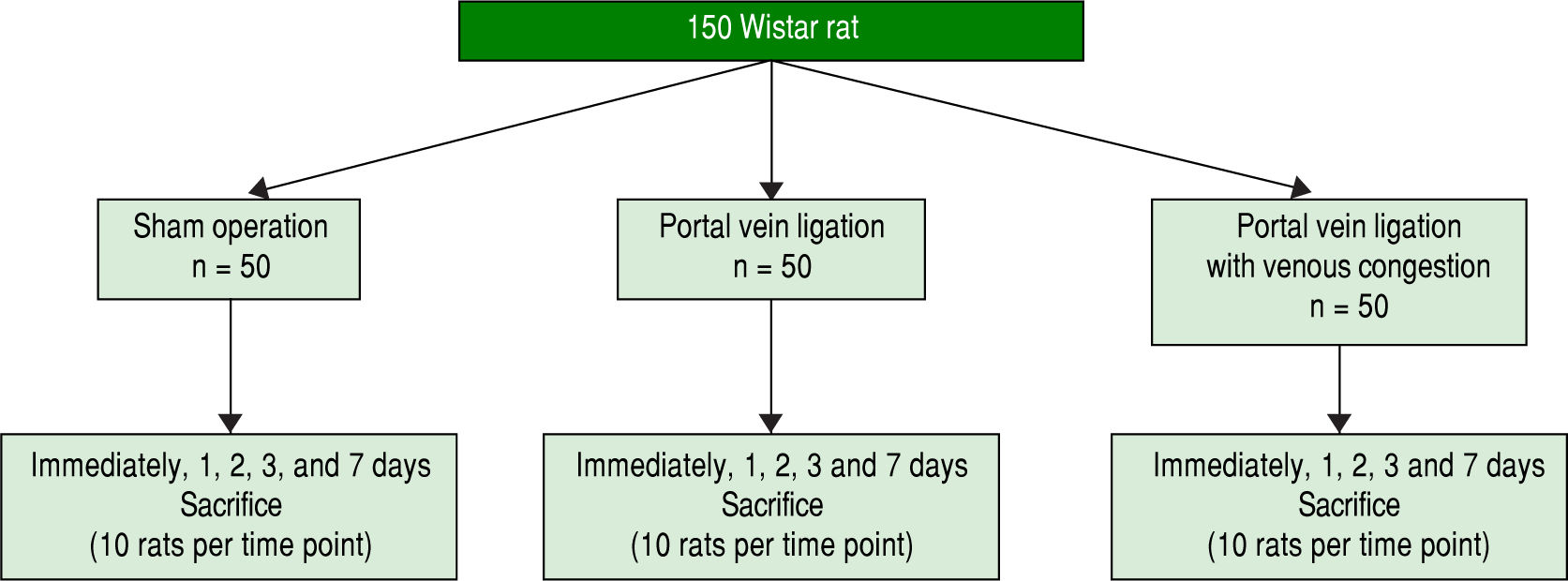
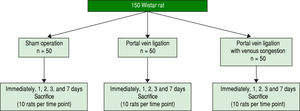
Experimental design and tissue allocationAnimals were weighed regularly. The 150 study rats were randomized into three groups of 50 each and sacrificed using a mixture of 4% isoflurane and saturated carbon dioxide atmosphere at different time intervals: immediately after surgery and 24 h, 48 h, 3 d, and 7 d later (10 rats per group at each time point) (Figure 2). At the time of killing blood samples were collected from the abdominal aorta, and the entire liver was removed and divided into the RML, LML, LLL, RL, and CL, each of which was weighed. Samples of the lobes then were processed for histologic examination. In particular, approximately 200 mg of liver tissue from the CL was fixed in 10% formalin solution or snap-frozen in liquid nitrogen, with the frozen samples stored at -80 ¯C until use.
To investigate the difference between PVL and PVL + C, the following variables were compared among the SHAM, PVL, and PVL groups.
Histologic and immunohistochemical examinationAfter immersion fixation in 10% formalin, liver tissues were embedded in paraffin and sectioned by routine methods. Some sections were stained with hematoxylineosin (HE). Additionally, the rate of liver regeneration was determined from the frequency of Ki-67-positive cells as follows. Liver sections from the CL taken at 24 h, 48 h, 3 d, and 7 d after surgery were deparaffinized and rehydrated with xylene and a graded ethanol series. Antigen retrieval was performed by placement of sections in 10-mM sodium citrate buffer (pH 6.0) for treatment in a microwave oven at 121 ¯C for 30 min. Endogenous peroxidase activity was quenched by immersion in 0.3% hydrogen peroxide in methanol for 30 min. Nonspecific binding sites then were blocked using a 10% rabbit serum solution for 15 min at 37 ¯C. Next, sections were incubated at room temperature for 1 h with a mouse monoclonal anti-rat Ki-67 antibody (DAKO Japan, Tokyo) diluted 1:25 in phosphate-buffered saline. Sections were then incubated with peroxidase-labeled anti-mouse antibody (Dakocytomation EnVision1 System) according to the manufacturer’s instructions, followed by incubation with 3,3’-diaminobenzidine and counterstaining with Mayer’s hematoxylin. After Ki-67-positive hepatocytes were counted in 10 randomly chosen high-power fields from three sections per group, means were calculated for analysis.
Hepatocytic apoptosis was assessed in sections from the LLL using the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method, using an in situ cell death detection kit (Roche Diagnostics, Tokyo, Japan) according to the manufacturer’s instructions. After TUNEL-positive hepatocytes were counted in 10 randomly chosen high-power fields from three sections per group, means were calculated for analysis.
All histologic analyses were performed without knowledge of the experimental group.
Blood laboratory testsSerum concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin (T.Bil) were measured using standard laboratory methods.
Real-time reverse-transcription polymerase chain reaction (RT-PCR)Cytokines that reportedly affected liver regeneration were assessed at the messenger RNA (mRNA) level determined by RT-PCR, which was performed in snap-frozen CL specimens obtained at 24 h, 48 h, 3 d, and 7 d after surgery. Expression of the following cytokines in the CL was investigated: tumor necrosis factor-a (TNF-a), interleukin-6 (IL-6), cyclin C, cyclin D1, hepatocyte growth factor (HGF), and interleukin-1aP (IL1-P). After total RNA was isolated from each sample using an RNAspin mini RNA isolation kit (GE Healthcare, Buckinghamshire, UK), complementary DNAs (cDNAs) were synthesized from 2 mg of each total RNA sample using high-capacity RNA-to-DNA kits (Applied Biosystems, Foster City, CA). Each cDNA sample was diluted 4-fold before PCR amplification. TaqMan gene expression assays (Applied Biosystems) guided the design of primers. RT-PCR was performed using a 7900HT Fast RT-PCR System (Applied Biosystems) and TaqMan Fast Advanced Master Mix (Applied Biosystems) according to the manufacturer’s instructions. Amplification consisted of an initial 10-min denaturation phase at 95 ¯C, followed by 40 cycles of denaturation at 95 ¯C for 10 s, annealing at 60 ¯C for 40 s, and extension at 72 ¯C for 10 s. Degree of expression of each target gene was normalized relative to expression of the mRNA encoding glyceraldehyde-3-phosphate dehydrogenase (GAPDH) determined in the same sample to permit informative between-sample comparisons. Assays were performed in triplicate.
Statistical analysisContinuous data are expressed as the mean (± standard deviation) or median (range), and were analyzed using the Mann-Whitney U test. The %2 test or Fisher’s exact test was used to analyze categorical variables. A two-sided P value < 0.05 was considered to indicate significance in all analyses.
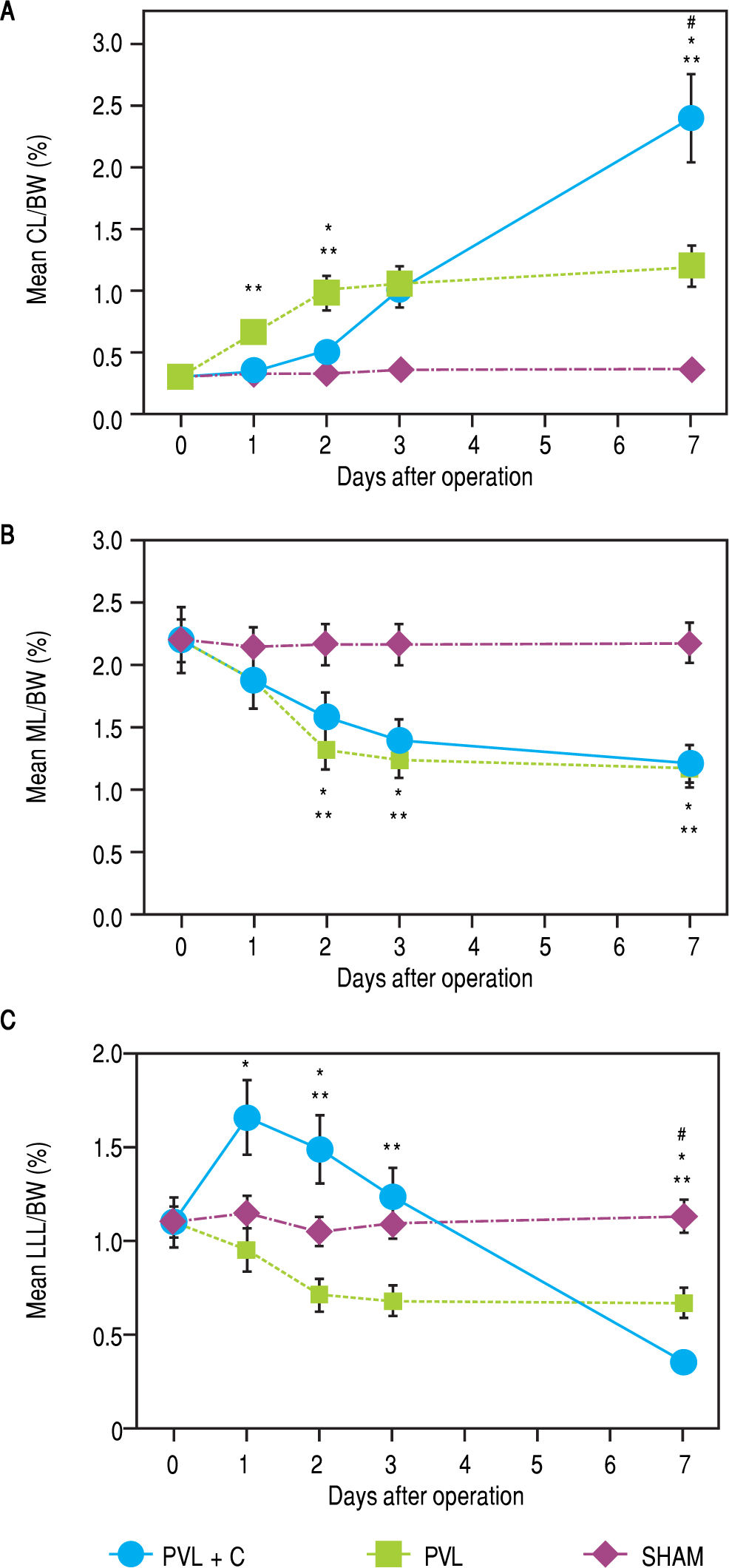
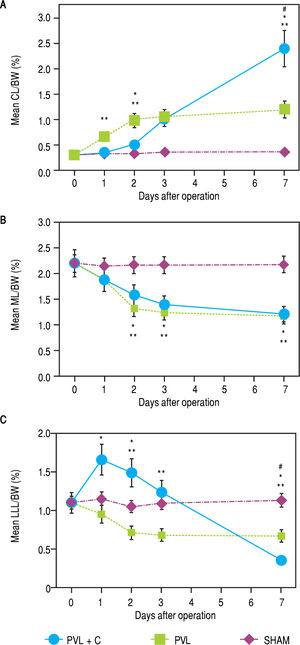
ResultsChronological changes in weight regeneration of each liver lobe after surgeryChronological changes in liver weight regeneration after surgery are shown in figure 3. The ratio of CL weight to body weight (CL/BW) continued to increase up to postoperative day (POD)7 in the PVL + C group; however, the growth of CL/BW terminated at POD2 in the PVL group and remained unchanged in the SHAM group. CL/ BW at POD7 was 0.31 ± 0.02% in the SHAM group, 1.22 ± 0.18% in the PVL group, and 2.41 ± 0.33% in the PVL + C group, respectively (SHAM vs. PVL, P < 0.01; PVL vs. PVL + C, P < 0.01; SHAM vs. PVL + C, P < 0.01). With regard to LLL, i.e., the embolized (congested) liver, LLL weight to body weight ratio (LLL/BW) continued to decrease up to POD7 in the PVL + C group but reached nadir at POD2 in the PVL group and remained unchanged in the SHAM group. LLL/BW at POD7 was 1.13 ± 0.15% in the SHAM group, 0.67 ± 0.08% in the PVL group, and 0.35 ± 0.03% in the PVL + C group (SHAM vs. PVL, P < 0.01; PVL vs. PVL + C, P < 0.01; SHAM vs. PVL + C, P < 0.01). In contrast, the weight regeneration of ML, which was embolized alone in the PVL or PVL+C group, showed no significant difference between the PVL and PVL + C groups (Figure 3). Similarly, chronological changes in weight regeneration of the RL, which was embolized alone in the PVL or PVL + C groups, did not show any significant difference between the PVL and PVL + C groups (data not shown).
Hepatic lobe weights as percentages of BW for CL (A), ML (B), and LLL (C) after SHAM, PVL, and PVL + C procedures. CL weights relative to body weights are significantly greater after PVL and PVL + C at postoperative days 3 and 7 than those for the SHAM group. This relative CL weight is significantly greater for the PVL + C than the PVL group at postoperative day 7. Values are expressed as mean ± standard deviation. #, P < 0.01 for the PVL + C group vs. the PVL group. *: P < 0.01 for the PVL + C group vs. the SHAM group. **: P < 0.01 for the PVL group vs. the SHAM group. PVL: portal vein ligation. CL: caudate lobe. LML: middle lobe. LLL: left lateral lobe. SHAM: sham operation. BW: body weight. PVL + C: portal vein ligation with venous congestion.
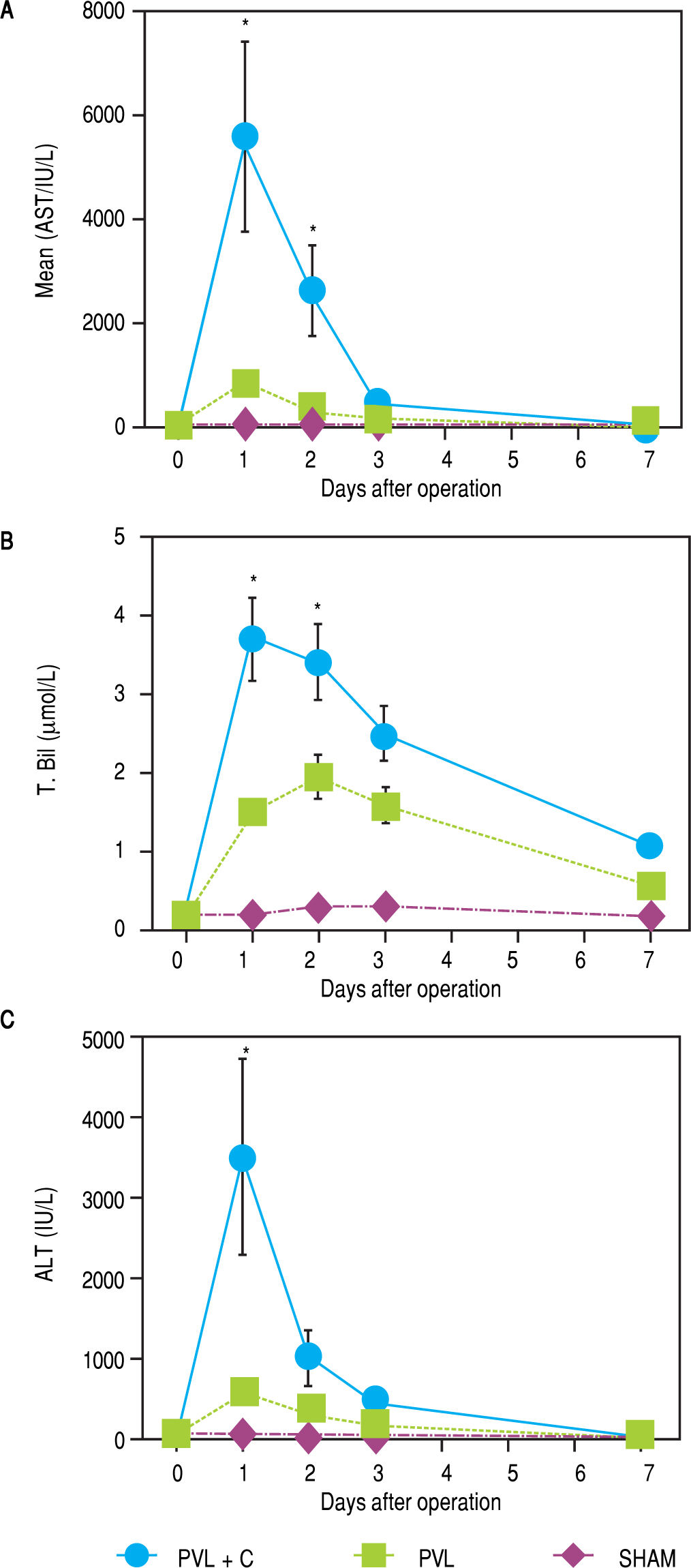
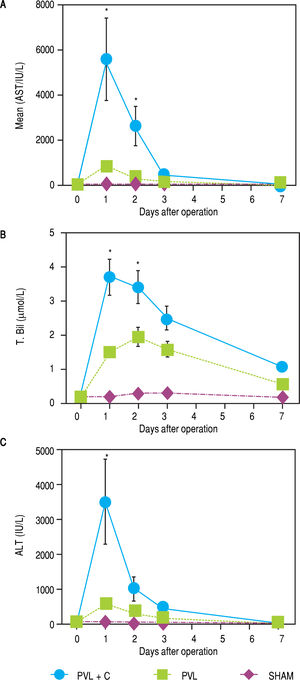
Chronological changes in serum ALT, AST, and T.Bil levels in each group are shown in figure 4. A remarkable elevation of these variables was observed at POD1 in the PVL + C group. A > 5-fold elevation in ALT and AST or > 2-fold elevation in T.Bil were observed in the PVL + C group in comparison with the PVL group at POD1 (P < 0.01). However, the PVL + C group values rapidly recovered and did not show any significant differences compared to the other groups at POD3 and thereafter.
Serial changes in postoperative liver function test results. AST, ALT, and T.Bil are significantly higher in the PVL + C than in the PVL group at 24 h postoperatively. Results are expressed as the median (range) of 10 rats per group at each time point. *: P < 0.01 for the PVL + C group vs. the PVL group. AST, aspartate aminotransferase. ALT: alanine aminotransferase. T.Bil: total bilirubin. PVL: portal vein ligation. PVL + C: portal vein ligation with venous congestion.
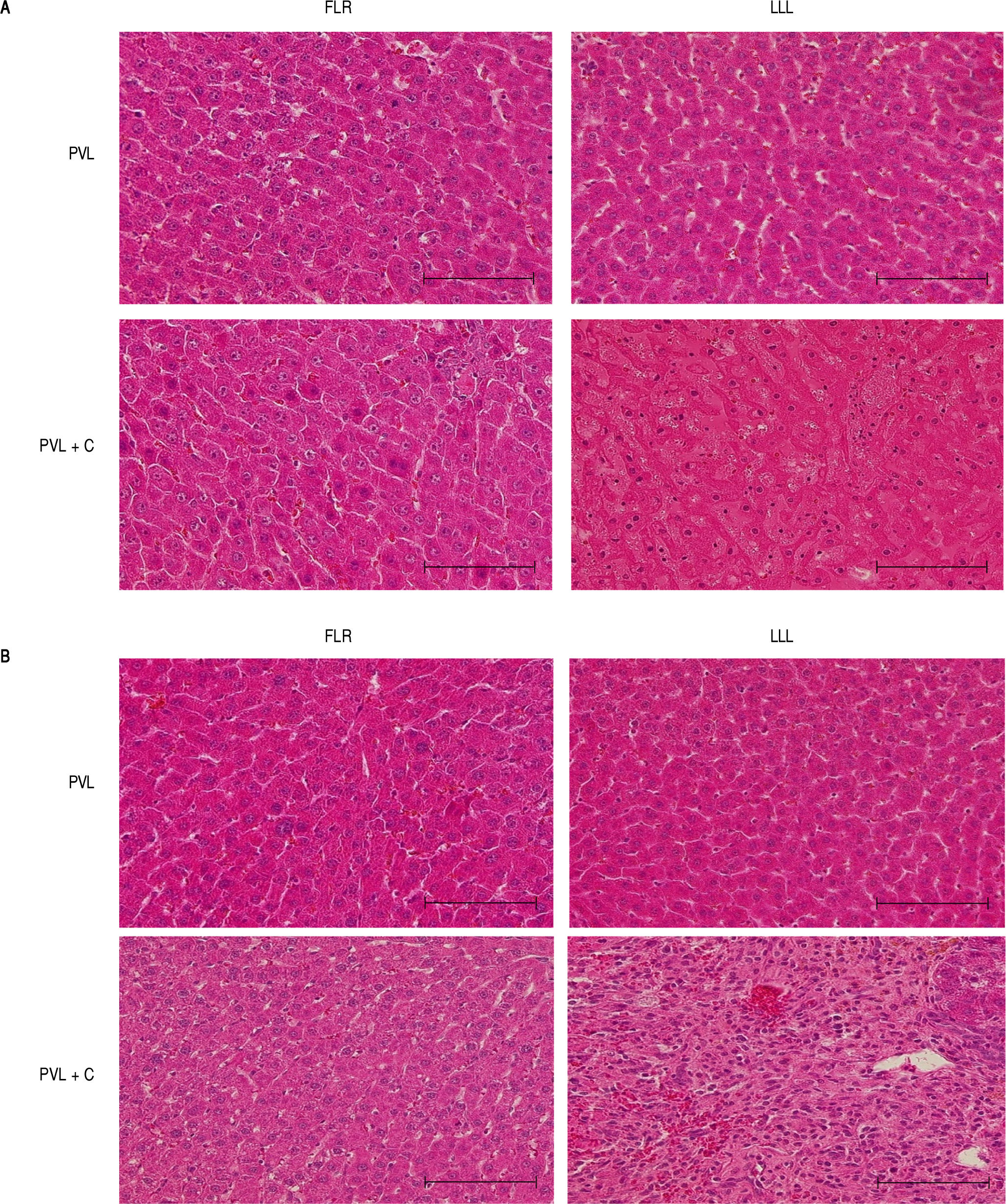
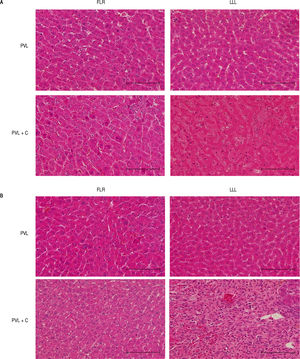
Representative photomicrographs at POD1 and POD7 (HE) are shown in figure 5. The most marked microscopic abnormalities at POD1 were seen in the LLL of the PVL+C group, including hepatocyte atrophy, sinusoidal congestion, and cytoplasmic vacuolation (Figure 5A), with fibrosis ensuing by POD7 (Figure 5B). Although these findings were observed after PVL alone, the grade of each was severer in the PVL + C group than in the PVL group. Furthermore, fibrosis was not observed at any time points in the PVL group.
Representative microscopic images of FLR and LLL for the PVL and PVL + C groups at 24 h and 7 dpostoperatively. A. In the PVL + C group, atrophy, sinusoidal congestion, and cytoplasmic vacuolation are observed in the LLL at 24 h. B In the same group, severe fibrosis is observed in the LLL at 7 d. Scale bar = 100 pm. (A) and (B): hematoxylin and eosin stain, original magnification x200. FLR: future liver remnant. LLL: left lateral lobe. PVL: portal vein ligation. PVL + C: portal vein ligation with venous congestion.
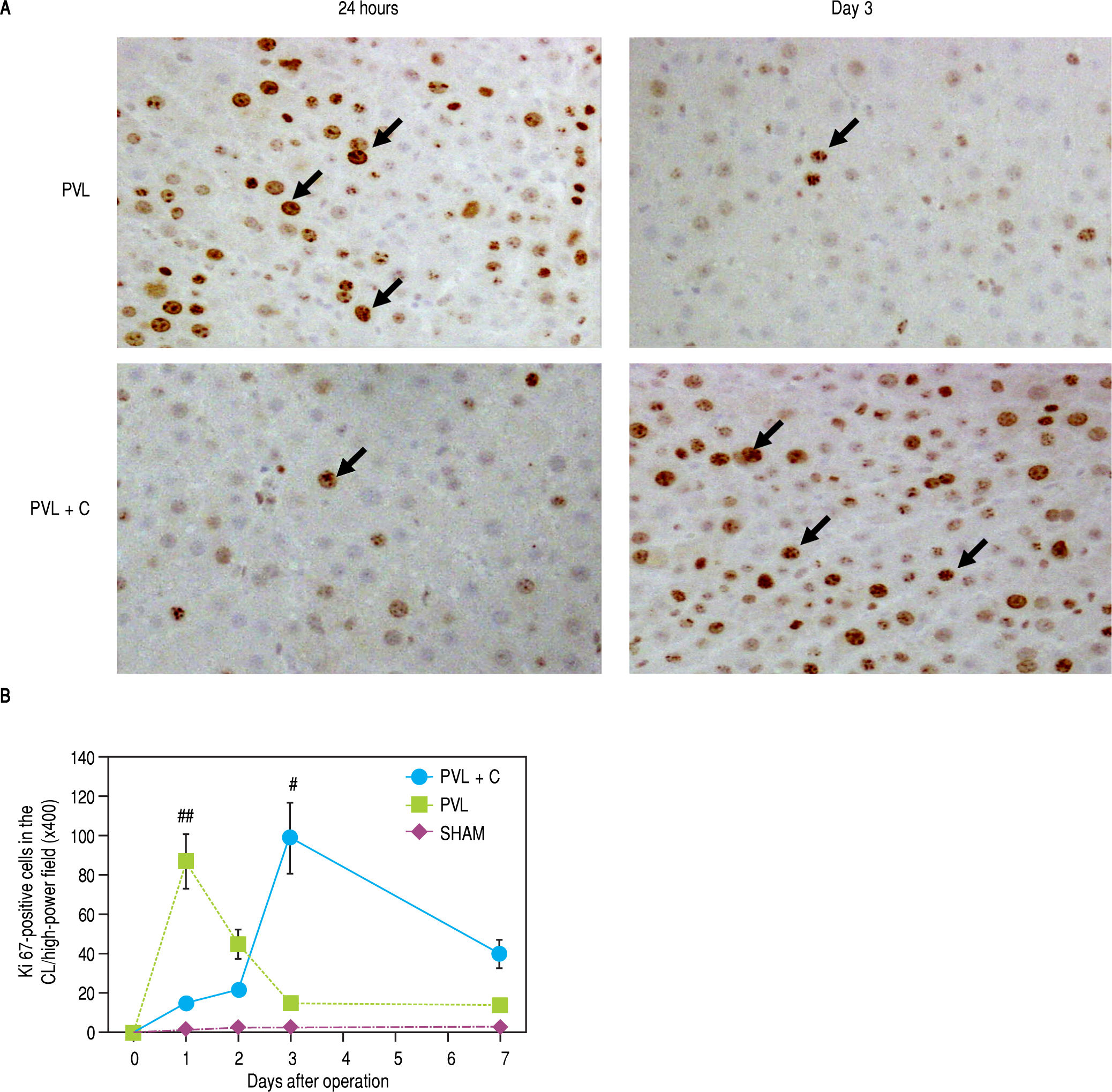
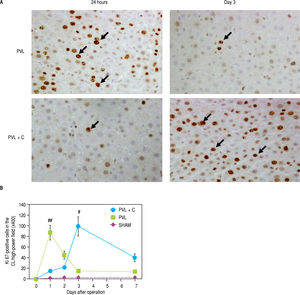
A comparison of Ki-67 expression in the CL between the PVL and PVL + C groups showed that the maximum Ki-67-positive hepatocyte counts during the 7 days after surgery were significantly greater in the PVL + C than in the PVL (PVL+C vs. PVL, P < 0.01; Figure 6A and Figure 6B). Furthermore, Ki-67 positive hepatocyte counts peaked at POD1 and thereafter decreased rapidly to the same level as that in the SHAM group in the PVL group, whereas Ki-67-positive hepatocyte counts increased up to POD3 and thereafter decreased gradually in the PVL + C group. Consequently, Ki-positive hepatocyte counts at POD7 were significantly greater in the PVL+C group than in the PVL group (40 ± 7 vs. 14 ± 3, P < 0.01). These findings suggest that regenerative stimulation of the CL caused by PVL+C is greater in magnitude and more prolonged in duration than that caused by PVL alone.
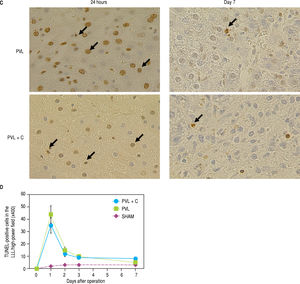
Ki-67-positive cells in the CL (A,B) and TUNEL-positive cells in the LLL (C,D) over time following surgery. A. Ki67 staining in CL tissue samples taken from PVL and PVL + C rats at 24 h and 3 d. Arrows indicate positive cells, B. The percentage of Ki-67-positive CL cells at postoperative days 3 and 7 is significantly higher in the PVL + C group than in the PVL group. *:P < 0.01 for the PVL + C vs. the PVL group. **: P < 0.01 for the PVL vs. the PVL + C group. CL: caudate lobe. PVL: porta vein ligation. PVL + C: portal vein ligation with venous congestion. TUNEL: terminal deoxynucleotidyl transferase dUTP nick-end labeling. LLL: left lateral lobe. SHAM: sham operation. C. TUNEL staining in the LLL tissue samples taken from PVL and PVL+C rats at 3 d. Arrows indicate positive cells. D. The percentage of TUNEL-positive cells is not significantly different between the PVL + C and PVL groups at any time point. #: P < 0.01 for the PVL + C vs. the PVL group. ##: P < 0.01 for the PVL vs. the PVL + C group. CL: caudate lobe. PVL: portal vein ligation. PVL + C: porta vein ligation with venous congestion. TUNEL: terminal deoxynucleotidyl transferase dUTP nick-end labeling. LLL: left lateral lobe. SHAM: sham operation.
Maximum counts of TUNEL-positive hepatocytes in the LLL were observed at POD1 in both the PVL and PVL + C groups. Furthermore, these counts did not differ between the two groups at any time point (Figure 6C and Figure 6D).
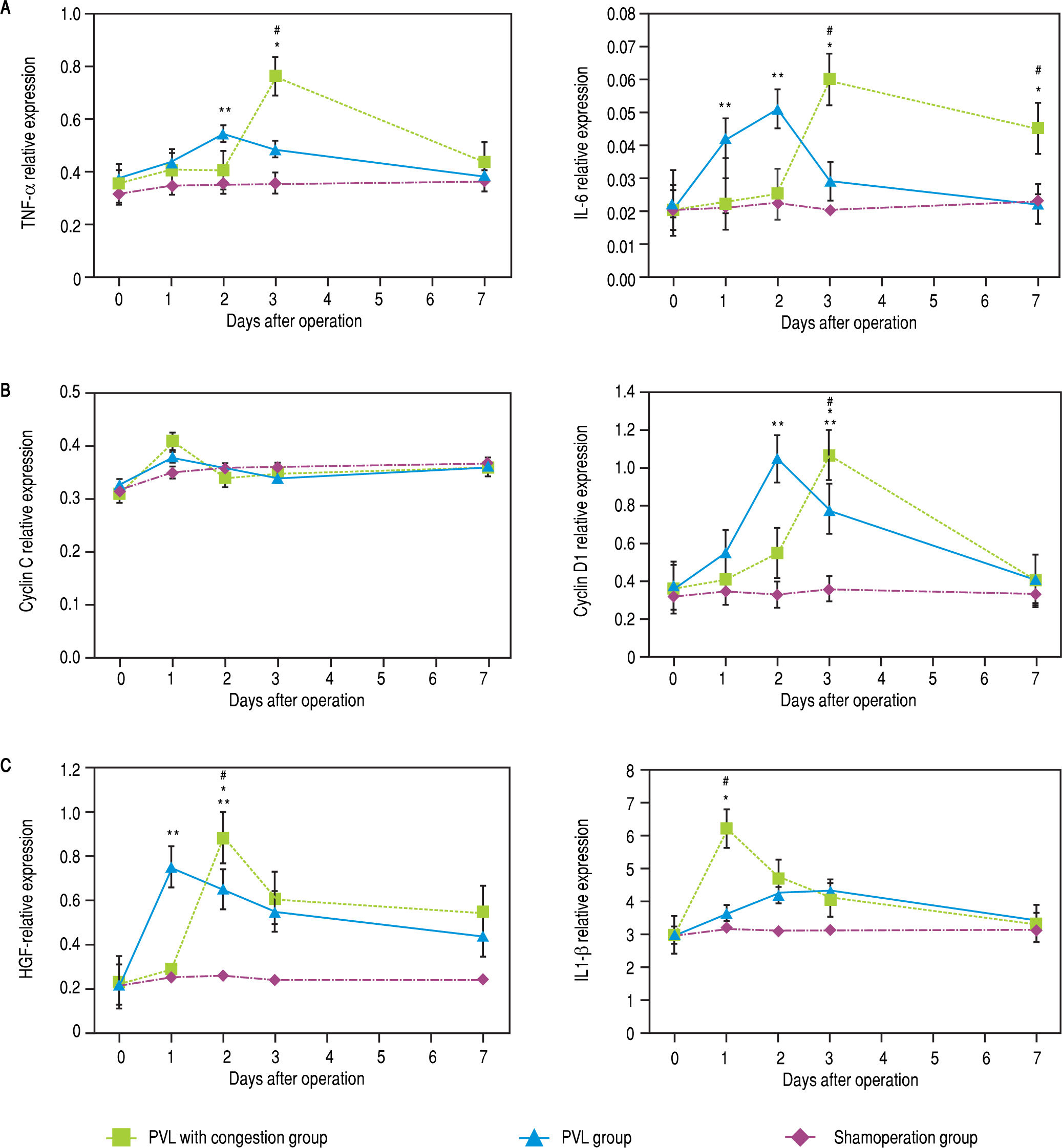
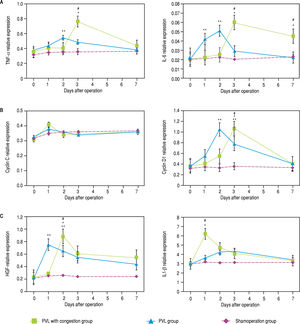
Up-regulation of the mRNA expression of pro-inflammatory cytokinesMolecular analysis of the FLR (represented by the CL) demonstrated larger increases in the TNF-a, IL-6, and cyclin D1 mRNA expression in the PVL than in the PVL + C group at 48 h after surgery, while expression of mRNA encoding IL1-0 was higher in the PVL + C group (P < 0.01, Figure 7A to C). Expression for IL1-P at 24 h, HGF at 48 h, and TNF-a, IL-6, and cyclin D1 on POD3 were higher in the PVL + C than in the PVL group (P < 0.01). On postoperative day 7, IL-6 expression was higher in the PVL + C than in the PVL group (P < 0.05). Cyclin C expression was increased at 24 h after surgery, but showed no significant difference between the two groups at that time point.
Expression of mRNAs for cytokines in CL tissue after the procedures. Expression was determined using quantitative real-time RT-PCR and standardized to GAPDH mRNA expression. A. Relative expression of TNF-a and IL6 mRNAs is signifcanty higher in the PVL than the PVL + C group at 48 h after surgery, while on postoperative day 3 these are signifcantiy higher in the PVL + C than the PVL group. B. Relatve expression of cyciin D1 mRNA is signifcanty higher in the PVL than PVL + C group at 48 h after surgery, while on postoperative day 3 this is signifcanty higher in the PVL+C than the PVL group. C. Relatve expression of IL1-β mRNA at 24 h and of HGFat 48 h is significantly higher in the PVL + C than the PVL group. #: P < 0.01 for the PVL + C vs. the PVL group. *: P < 0.01 for the PVL + C vs. the SHAM group. **: P < 0.01 for the PVL vs. the SHAM group. mRNA: messenger RNA. CL: caudate lobe, PVL: porta vein ligafon. PVL + C: porta vein ligation with venous congesfon. SHAM: sham operafon. RT-PCR: reatme reverse-transcription polymerase chan reaction. GAPDH: glyceraldehyde-3-phosphate dehydrogenase. TNF-α, tumor necrosis factor-a. IL-6, interleukin-6. HGF: hepatocellular growth factor, IL1-β, interleukin-lβ.
To summarize these findings, a greater and more prolonged expression of TNF-a and IL-6 were observed in the PVL + C group than in the PVL group. Furthermore, a greater magnitude of maximum expression of HGF and IL1-P was observed in the PVL + C group than in the PVL group. However, a difference in the maximum expression of cyclin C or cyclin D1 was not observed among the groups, although the expression of cyclin D1 in the PVL + C group was delayed. A significant difference in cyclin D1 expression between the PVL and PVL + C groups was observed at POD2 or POD3, but not on the other days.
DiscussionTo the best of our knowledge, there have been no reports of an animal model to investigate the beneficial effect of congestion in addition to PVE/L; therefore, we developed a rat model of PVL + C. Our model showed that a greater FLR expansion and more severe atrophy of the embolized/congested lobe were achieved by PVL + C compared with PVL alone. Hence, we considered that the findings obtained from our rat model could be reflected in the clinical setting.
In the present study, we corroborated that PVL + C caused severe devastation of the planned resected liver than PVL alone, and further demonstrated that the expression of several mediators promoting liver regeneration in FLR were greater and/or more prolonged in the PVL + C group than in the PVL group.
Increased devastation of the LLL in the PVL+C group compared to the PVL group was confirmed not only quantitatively but also qualitatively. A reduction in the LLL weight as well as histologically-proven impairment including sinusoidal congestion, cytoplasmic vacuolization, and fibrosis were more severe and sustained in the PVL + C than in the PVL. Furthermore, significantly higher values of the results from the serum liver function test were considered to reflect the severer qualitative devastation of the LLL caused by congestion in addition to PVL. The functional demand of the devastated part of the liver was reported to shift to another portion.15 This functional shift was considered to serve as regenerative stimulation to the intact liver. Therefore, the more severe the devastation of the embolized liver, the larger the FLR. PVL reportedly promotes hepatocyte apoptosis in the embolized liver.15 However, hepatocyte apoptosis in the LLL assessed by TUNEL staining did not show any difference between the PVL and PVL + C groups in the present study. Thus, the increased devastation caused by congestion in addition to PVE/L was brought on by factors other than apoptosis. On the other hand, several clinical studies have demonstrated that hepatic vein embolization combined with PVE/L and the ALPPS procedure increased the mortality and/or morbidity rates compared with PVE/L alone. In our model, venous congestion was applied to only one of the four embolized liver lobes. Nevertheless, a > 5-fold elevation in the serum transaminase level was observed in the PVL + C group compared with the PVL group. In clinical settings, hepatic vein embolization combined with PVE/L and the ALPPS procedure affects all embolized liver lobes. Therefore, a more pronounced hepatic parenchymal injury might be considered to occur in the clinical setting than in our model, leading to more frequent mortality and/ or morbidity than with PVE/L alone.
With regards to the chemical mediators affecting liver regeneration, we examined the mRNA expression of TNF-a, IL-6, HGF, cyclin C, cyclin D1, and IL1-P in the FLR to explore the molecular mechanisms of regeneration in the CL. These cytokines have been reported to be important for hepatocyte proliferation and are thus considered suitable for investigating the possible mechanisms of the beneficial effect of congestion in addition to PVE/L, as well as assessing the reliability of our model.16 The mRNA expression of TNF-a, IL-6, and HGF were up-regulated significantly in the PVL + C group compared to the PVL group, but not in the SHAM group. The cytokines, including TNF-a, IL-6, and HGF, act as mitogenic stimuli on hepatocytes during regeneration.16,17 TNF-a and IL-6 are particularly important in initiating liver regeneration. Produced by activated Kupffer cells, these pro-inflammatory cytokines promote transition of hepatocytes from the G0 to the G1 phase.18-20 Our findings indicate that venous congestion in addition to PVE/L caused increased expression and/or a more prolonged up-regulation of these pro-inflammatory cytokines than PVE/L alone. Interestingly, PVL+C resulted in a delayed upregulation of cyclin D1 mRNA compared to PVL alone, even though the maximum magnitude of cyclin D1 expression did not differ among the PVL and PVL + C groups. This important cell-cycle regulatory protein can stimulate cell passage through the G1 checkpoint into the S phase.21 As previously reported, liver regeneration is a changing process consisting of multiple intracellular events that show complex interactions involving cytokines and growth factors.16,22 Our findings are consistent with such a process and suggests that venous congestion induces changes in cytokines and regulatory proteins that contribute to the increase in FLR volume after PVL+C. Previous studies have reported that high-mobility group protein 1 (HMGB-1) can stimulate inflammatory cytokines in response to internally detected biologic threats.23 As such, many details of the mechanisms by which venous congestion increases cytokine expression remain unclear. We believe that the present rat model of PVL+C could be useful for future studies to clarify these complex relationships between venous congestion and inflammation.
The present study has several limitations. Firstly, our model does not include a liver partition procedure, although it was designed with the aim to investigate the additional effect of hepatic vein embolization as well as ALPPS to PVE/L alone. The liver partition procedure itself is considered to produce a stimulatory effect for liver regeneration.24 Unfortunately, the effect of liver partition procedure itself cannot be assessed by our model. Second, the volume of intact liver in our model may be too large compared to that in a clinical setting. Our rat model of PVL + C was designed to simulate a 90% hepatectomy in rats. As we previously reported, rats can survive a 90% hepatectomy, in which all hepatic lobes except for entire CL are removed.25 In the clinical setting, hepatic vein embolization combined with the PVE/L or ALPPS procedure is usually applied for patients who are considered unlikely to survive surgery because of a very small FLR and/or insufficient FLR expansion. Therefore, we should have generated a fatal hepatectomy model, such as a 95% hepatectomy model, in which all hepatic lobes except for the posterior CL are removed as rats cannot survive this surgery, as previously reported.25 However, a PVL model to simulate a 95% hepatectomy in rats requires separate ligation of the anterior CL portal branch in addition to the RL, RML, LML, and LLL branches. Separate isolation of the anterior CL portal branch is quite difficult because divergence of the anterior and posterior CL portal branches is almost always entirely buried in the hepatic parenchyma in rats. Therefore, we did not simulate a 95% hepatectomy. Third, we did not assess cytokine profiles within 24 h after surgery. Several previous reports have shown that expression of mediators affecting liver regeneration is initiated immediately after surgery, within several hours.26,27 However, the present study results suggest that congestion in addition to PVL causes greater and more prolonged expression of these mediators than PVL alone, which we consider provides evidence for a difference between PVL + C and PVL alone. Even if these limitations, we believe that the present study provides important new information concerning the significance of congestion in addition to PVL for stimulating FLR regeneration.
In conclusion, the PVL + C rat model was considered useful to investigate the beneficial effect of congestion in addition to PVC. PVL + C resulted in more severe devastation of the embolized liver, as well as increased and more prolonged expression of factors promoting liver regeneration in the non-embolized liver compared with PVL alone, leading to earlier and more robust FLR regeneration.
Abbreviations- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
BW: body weight.
- •
CL: caudate lobe.
- •
FLR: future liver remnant.
- •
HE: hematoxylin-eosin.
- •
HGF: hepatocyte growth factor.
- •
HMGB-1: high-mobility group protein 1.
- •
IL-1p: nterleukin 1p.
- •
GAPDH: glyceraldehyde-3-phosphate dehydrogenase.
- •
IL-6: interleukin 6.
- •
LLL: left lateral lobe.
- •
LML: left middle lobe.
- •
mRNA: messenger RNA.
- •
PVE: portal vein embolization.
- •
PVL: portal vein ligation.
- •
PVL + C: PVL with venous congestion.
- •
PVO: portal vein occlusion.
- •
RL: right lobe.
- •
RML: right middle lobe.
- •
RT-PCR: real -time reverse-transcription polymerase chain reaction.
- •
SHAM: sham operation.
- •
T.Bil: total bilirubin.
- •
TNF a: tumor necrosis factor-a.
- •
TUNEL: terminal deoxynucleotidyl transferase dUTP nick-end labeling.
The authors declares that there is no conflict of interest regarding the publication of this article.
Compliance with Ethical RequirementsAll institutional and national guidelines for the care and use of laboratory animals were followed.