Administration of nonselective beta-blockers in prophylaxis of first variceal bleeding is not suitable for all patients. Thus, we evaluated endoscopic variceal band ligation (EVBL) in primary prevention of bleeding in patients with cirrhosis and large esophageal varices. A total of 73 consecutive patients with liver cirrhosis and large esophageal varices without a history of gastrointestinal bleeding were randomized to receive either EVBL or propranolol and were followed for up to 18 months. Forty patients underwent EVBL and 33 patients received propranolol. Variceal bleeding occurred in 2 patients in the EVBL (5%) and in 2 patients in the propranolol group (6%, NS). The 18 month actuarial risk for first variceal bleed was 5% in the EVBL (95% CI, 0-12%) and 20% in the propranolol group (95% CI, 0-49%, NS). The actuarial probability of death at 18 months of follow-up was 5% (95% CI, 0-11%) in the EVBL group and 7% (95% CI, 0-17%, NS) in the propranolol arm. In conclusion, EVBL was an effective and safe alternative to propranolol in primary prophylaxis of bleeding in patients with large esophageal varices.
Esophageal variceal bleeding is one of the most serious complications of liver cirrhosis with portal hypertension. The risk of bleeding varies between 835% within 2 years of follow up.1-3 The risk of first variceal bleeding is related to the size of varices, degree of liver dysfunction (Child-Pugh score), elevated hepatic venous wedge pressure gradient (HVPG) and the presence of mucosal red signs detected during endoscopy.2 Despite the fact that mortality has been significantly reduced over the past 2 decades thanks to advanced intensive care, vasoactive drugs and endoscopic therapy, the 6-week mortality rate is still very high (17.5%).4 Primary prophylaxis of first variceal bleeding is therefore an important therapeutical goal.
Several randomized studies and meta-analyses have shown that nonselective β-blockers (propranolol, nadolol) decrease the risk of bleeding by 4050% as compared to placebo with a trend toward improved survival.2,5,6 However, the use of β-blockers has several shortcomings. The treatment is associated with numerous side effects in up to 40% of patients7 and reduction in portal pressure is not adequate to prevent bleeding in 30-40% of patients (HVPG < 12 mm Hg or a reduction by 20%).8 Also, rebound phenomena with an increased risk of bleeding following sudden cessation of therapy9 make lifelong therapy necessary in the majority of patients. Contraindications preclude the use of beta-blockers in about 15% of the patients who are considered for this treatment.10 No other alternatives to non-selective b-blockers exist for pharmacological prophylaxis of variceal bleeding in routine practice.
Endoscopic therapy, either endoscopic variceal band ligation (EVBL) or sclerotherapy, are highly effective in both the management of acute variceal bleeding and secondary prophylaxis.11 Sclerotherapy is not recommended for primary prophylaxis because of inconsistency of results across trials.12 EVBL has been shown to decrease the relative risk of first bleeding by 64% and mortality by 45% when compared to no treatment.13 EVBL has been compared to beta-blockers in several trials with inconsistent results.14-19 EVBL was superior to beta-blockers in two randomized trials,20-21 but a reduction in mortality was observed in only one of them.21 Furthermore, two prospective control trials were prematurely terminated for different reasons, one because of excess bleeding in the ligation group, the other for high treatment failure in the propranolol group.21-22 The latest trial reported a lower rate of esophageal variceal bleeding in the EVBL patients.23 Thus, the results of the available studies must be interpreted with caution, and a recent consensus workshop recommends EVBL be offered to patients with medium or beta-blockers instead of beta-blockers large varices and contraindications or intolerance to beta-blockers.24
To further elucidate the role of EVBL in primary prophylaxis of variceal bleeding, we conducted a prospective, randomized, multicenter trial designed to compare EVBL with propranolol treatment.
Materials and MethodsThe study design is depicted in Figure 1Seventy-three consecutive patients with portal hypertension due to liver cirrhosis with large esophageal varices were enrolled at six participating university hospitals. Written informed consent was obtained from all patients according to the guidelines of the 1975 Declaration of Helsinki. The local ethics committees and the Czech Institute for Drug Control approved the study protocol. The diagnosis of liver cirrhosis was established on the basis of clinical, ultrasonographic and biochemical examinations. If necessary, liver biopsy was performed to confirm the diagnosis. The severity of liver disease was classified according to the Pugh modified Child’s score. The size of varices (> 5 mm) was assessed by a comparison to an opened biopsy forceps. Exclusion criteria were as follows:
- 1.
Non-cirrhotic cause of portal hypertension.
- 2.
History of gastrointestinal bleeding of any origin.
- 3.
History of sclerotherapy, EVBL or portosystemic shunt.
- 4.
Documented malignant disease.
- 5.
Gastric or duodenal ulcer.
- 6.
Congestive heart failure.
- 7.
Chronic renal insufficiency with plasma creatinine ≥ 150 μmol/l.
- 8.
Treatment with β-blockers, nitrates, ACE inhibitors or verapamil within 2 months before randomization.
- 9.
Concomitant antiviral therapy.
- 10.
Hypersensitivity to propranolol.
- 11.
Atrioventricular block of grade II and III, sick-sinus syndrome, bradycardia under 60 beats/minute.
- 12.
Asthma or chronic obstructive airway disease.
- 13.
Decompensated diabetes mellitus.
- 14.
Pregnancy or lactation.
Randomization to EVBL or propranolol therapy was done at each center using a computer-generated table of random numbers.
Patients were followed for up to 18 months or until liver transplantation or death. The primary end point was the number of variceal bleeding episodes. Secondary end points were:
- 1.
Death due to variceal bleeding, underlying liver disease or any other cause.
- 2.
Gastrointestinal bleeding from any other origin than esophageal varices.
- 3.
Evaluation of portal hypertensive gastropathy.
- 4.
Side effects and complications of EVBL and propranolol.
- 5.
Recurrence of varices in the EVBL study arm.
Patients assigned to the EVBL group underwent ligation after an initial screening gastroscopy that was performed to assess the size and appearance of esophageal and gastric varices and portal hypertensive gastropathy (PHG) and to exclude other lesions such as ulcers and tumors. The intravenous administration of 5 to 10 mg of diazepam or 2.5 to 5 mg of midazolam was used for sedation on individual basis. EVBL was performed within 1 week of randomization using a multiband ligation device (Six-shooter, Wilson-Cook Inc., Winston-Salem, NC) and up to 6 bands were placed per session beginning in the distal esophagus just above the gastroesophageal junction. Procedures were performed at 2 week intervals until esophageal varices were eradicated. Variceal eradication was defined as a complete disappearance of varices or the presence of a varix being too small to be ligated. The interval between sessions was prolonged by 1 week if post-ligation ulcers were present. EVBL was performed by 1 or 2 experienced endoscopists at each center who had performed at least 20 EVBLs prior to this study. All side effects were carefully recorded. Patients were administered 1g of sucralfat emulsion 4 times per day on an individual basis until esophageal varices were eradicated. EVBL was continued in patients with recurrence, defined as the presence of a varix larger than 5 mm in diameter after initial eradication.
Propranolol therapyPatients were started on propranolol therapy (Pro- pra 40 von ct, Arzneimittel GmbH, Germany) within one week of randomization and the initial evaluation gastroscopy. During the initial visit, patients underwent baseline heart rate and blood pressure measurements and electrocardiography after 15 minutes of rest in a horizontal position. The starting dose for all patients was 20 mg twice daily. Patients were advised to take the evening dose before bedtime. The dose of propranolol was adjusted in 20-40 mg increments at weekly intervals, to achieve a reduction of the baseline heart rate by 25%, however not to decrease below 55 beats/min. All measurements were done under the same conditions. In case of side effects, heart rate < 55 beats/min or systolic blood pressure < 80 mmHg, propranolol dosage was reduced by 20 to 40 mg/day. Compliance was carefully assessed by a pill count and by pulse rate measurements.
Follow-upEVBL was performed until varices were eradicated and dose adjustments in the propranolol arm were performed until beta-blockade was adequate. All patients were seen in clinic every 3 months for clinical and biochemical examinations and an upper GI endoscopy was performed at 6 month intervals over a follow up time of up to 18 months. Bleeding from esophageal varices was defined as hematemesis and/or melena and the presence of endoscopic signs of bleeding as previously published (24). Bleeding was considered to be caused by portal hypertensive gastropathy (PHG) if distinct lesions of the gastric mucosa were present without evidence of bleeding from varices. Any death within 30 days after a bleeding episode was considered to be bleeding related.
Statistical AnalysisWe hypothesized a 20% reduction of bleeding incidence in the EVL group based on evidence available at the time of study design.20 Thus, assuming a power of 0.8 and alpha of 0.05, 44 patients were needed to be included in each study arm. An interim analysis was planned after recruiting 75% of the patients. Data were analyzed on an intention-to-treat basis. The variables between the two arms were compared using the non-paired t-test, Chi-square test, Fisher’s exact test or approximation based on arcsine transformation for comparison of the two relative frequencies (when appropriate). The actuarial probabilities of variceal bleeding and death were calculated by the Kaplan-Meier method and a comparison made using the long-rank test. The meta-analysis was performed as previously described.25 A fixed effects meta-analysis model was applied for combining estimates of a treatment difference across two trials. A two-tailed p < 0.05 was required for statistical significance. Analyses were performed using MedCalc (MedCalc Software, Mariakerke, Belgium).
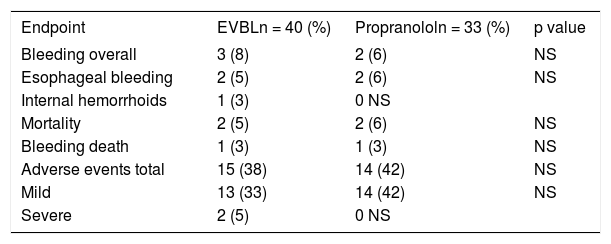
ResultsA total of 73 patients who met the inclusion criteria were randomized to the EVL (N = 40) and propranolol (N = 33) arms. The participating centers each enrolled 7 to 18 patients. Both groups were well matched with regard to age, etiology, endoscopic findings, severity of liver disease, and presence of ascites (Table 1). Forty-six patients (63%) had a diagnosis of alcoholic liver cirrhosis. Two patients were lost to follow-up (one from each group) and one patient underwent a liver transplantation 14 months after randomization. The main outcomes are summarized in Table 2
Baseline patient characteristics.
| EVBLn = 40 | Propranololn = 33 | |
|---|---|---|
| Age (yr) | 57 ± 9 | 56 ± 10 |
| Sex | ||
| Male (%) | 24 (60) | 27 (82) |
| Female(%) | 16 (40) | 6 (18) |
| Etiology of liver cirrhosis | ||
| Alcoholic (use of ETOH) | 6 | 2 |
| Alcoholic (without use of ETOH) | 20 | 18 |
| Autoimmune | 1 | 1 |
| Viral | 4 | 5 |
| Cholestatic | 6 | 4 |
| Others | 3 | 3 |
| Variceal red signs | ||
| No signs | 23 | 19 |
| Cherry red spots | 13 | 13 |
| Red wale markings | 2 | 0 |
| Hemocystic spots | 5 | 1 |
| Gastric varices before | 11 (28) | 7 (21) |
| treatment (%) | ||
| Portal gastropathy before | ||
| treatment | ||
| None (%) | 9 (23) | 10 (30) |
| Mild (%) | 27 (67) | 21 (64) |
| Severe (%) | 4 (10) | 2 (6) |
| Child-Pugh's score | ||
| A (%%) | 18 (45) | 20 (61) |
| B (%%) | 20 (50) | 10 (30) |
| C (%%) | 2 (5) | 3 (9) |
| Ascites(%) | 13 (33) | 7 (21) |
| Median of follow-up (months) | 11 (1-18) | 10 (1-18) |
Endpoints of the study.
| Endpoint | EVBLn = 40 (%) | Propranololn = 33 (%) | p value |
|---|---|---|---|
| Bleeding overall | 3 (8) | 2 (6) | NS |
| Esophageal bleeding | 2 (5) | 2 (6) | NS |
| Internal hemorrhoids | 1 (3) | 0 NS | |
| Mortality | 2 (5) | 2 (6) | NS |
| Bleeding death | 1 (3) | 1 (3) | NS |
| Adverse events total | 15 (38) | 14 (42) | NS |
| Mild | 13 (33) | 14 (42) | NS |
| Severe | 2 (5) | 0 NS |
NS: Not Significant.
For patients in the EVBL group, a mean of 2.2 ± 1.2 (SD) endoscopic sessions was needed to achieve variceal eradication (median 2, range 1-6) over a mean duration of 33 ± 19 days. Initial variceal eradication required placing a mean of 11 ± 6 rubber bands (median 12, range 4-34). Varices were successfully eradicated in all EVBL patients. The only serious side effects were the development of 2 deep ulcers that resulted in hemorrhage in two patients. No other complications except superficial post-banding (or residual) ulcers (40 patients, 100%), and transient retrosternal pain (13 patients, 32.5%) were detected (Table 3).
A total of 3 patients had bleeds in the EVBL arm (Table 2). Two patients bled from ligation-induced ulcers on esophageal varices that occurred within the first months of the study prior to variceal eradication. One originated from a deep post-ligation ulcer and was successfully treated with aethoxysclerol. In the other patient, variceal ulcer bleeding was detected post mortem as a cause of sudden death. No bleeds were documented after variceal obliteration. The third patient developed severe bleeding from internal hemorrhoids eight months after follow-up that required surgical suture. One patient died 3 months after randomization due to surgical complications of an elective cholecystectomy.
Variceal recurrence rate was evaluated 6 months after initial obliteration was achieved. A total of 4 patients (11.1%) developed recurrent varices at 6, 9 and 12 months after randomization. After the second eradication by EVBL, no variceal recurrence was detected. PHG was evaluated in 24 patients after 12 months of follow-up and compared with baseline. In 13 patients (54.2%), the degree of PHG did not change. It worsened in 3 patients (12.5%), and improved or disappeared in 8 cases (33.3%).
In patients treated with prophylactic propranolol, the mean daily dose required to reach an adequate β-blockade was 66.7±30.6 mg (median 80 mg, range 20-160 mg). The mean time required to achieve this blockade was 26.3 ± 13.8 days. All but four patients (12.1%) had their resting heart rate reduced by 25% from baseline. There were no serious adverse events ascribed to propranolol treatment. Fourteen patients (42 %) reported one or more mild side effects, including weakness (10 patients, 30.3%), dizziness (4 patients, 12.1%), nausea (2 patients, 6.1%) and insomnia (2 patients, 6.1%). Headache, bradycardia and decompensated liver cirrhosis occurred in single patients. More than one side effect was detected in 5 patients (Table 4.). After dosage adjustment, no patient on propranolol had to be withdrawn from the study due to side effects or confirmed incompliance.
A total of 2 deaths occurred in the propranolol arm (Table 2). Uncontrollable variceal bleeding leading to death occurred 2 months after randomization in a patient with an adequate reduction of heart rate. The second death was related to hepatorenal syndrome in a patient with continuous alcohol abuse after 5 months of propranolol treatment. Thirteen months after randomization, one patient bled from esophageal varices and gastric antral vascular ectasia (GAVE). The patient was successfully treated with sclerotherapy and argon plasma coagulation administered in 4 sessions.
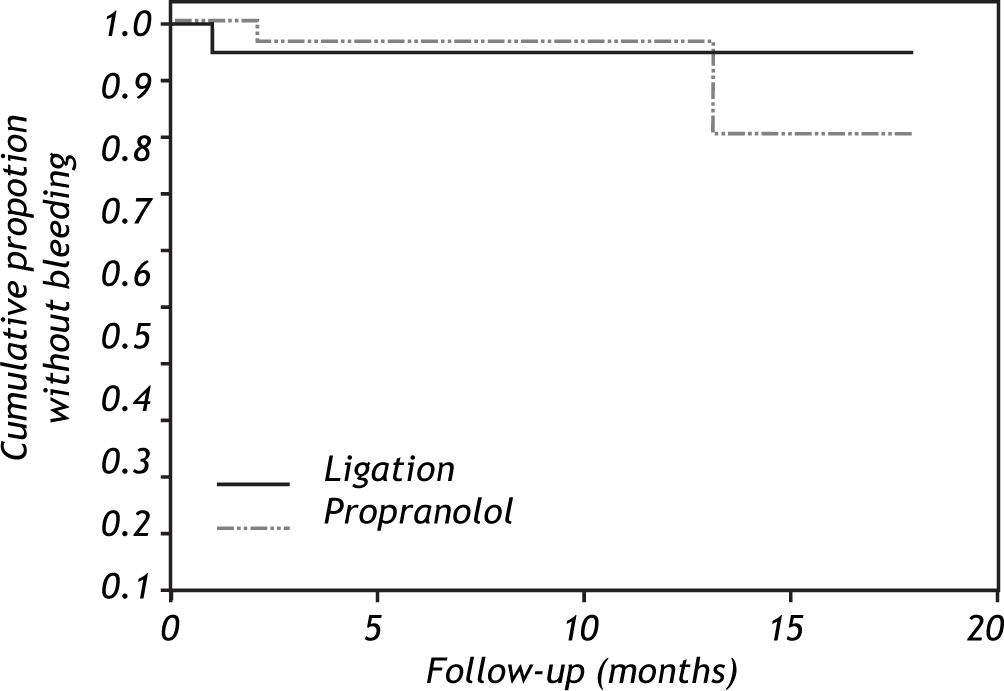
The actuarial risks of variceal bleeding at 18 months were 5% in the ligation arm (95% CI, 012%) and 20% (95% CI, 0-49%) in the propranolol group (Figure 2, p = 0.66, log-rank test). The actuarial probability of death at 18 months of follow-up was 5% (95% CI, 0-11%) in the ligation group and 7% (95% CI, 0-17%) in the propranolol arm (Figure 3, p = 0.83, log-rank test).
The planned interim analysis, performed on an intention-to-treat basis, revealed no statistical significant difference in first variceal hemorrhage, overall and bleeding-related mortality between the groups. Sample size recalculation showed that it would not be possible to demonstrate a significant difference between treatments with the originally planned number of patients. For this reason, the study was terminated.
To increase the power of our data, a meta-analysis of our study and the most recently published study with a similar design was performed.23 The study of Perez-Ayuso was similar in size, inclusion criteria and disease severity of its patients. The analyzed groups did not significantly differ in size, gender, etiology of disease, Child Pugh‘s score and presence of ascites. A test for heterogenity across the studies was not significant for all presented variables. When the endpoints were compared, there was no difference in mortality and overall as well as esophageal bleeding (Table 5).
Primary prophylaxis of first variceal bleeding is a standard of care in patients with liver cirrhosis and large esophageal varices, however discussion continues as to which treatment modality is superior. We compared EVBL with propranolol treatment for the prevention of first variceal bleeding in our randomized, controlled, multicenter trial, and found no significant difference between the two regimens with respect to variceal bleeding and mortality.
The results of our study are in agreement with the majority of prior studies, indicating that prophylactic ligation does not significantly differ from β-blockers in respect to the risk of first bleeding and survival rate.14-19 However, there are studies where a significant benefit in favor of EVBL was found. The trial by Jutabha, et al.21 was stopped after an interim analysis showed a significantly higher failure rate in propranolol-treated patients. This result was mainly attributable to the fact that no bleeding occurred in the EVBL group. Such good banding results would be unexpected even with the latest equipment in expert hands. The actuarial probability of bleeding (43%) after 18 months in the propranolol group reported by Sarin et al. was higher than that observed by other authors.20
EVBL therapy appeared to have some advantages in our study; variceal obliteration was achieved very rapidly, on average in two endoscopic sessions, with the use of an average of 10 rubber bands. Such a rapid eradication may be useful, especially in patients with low compliance. Furthermore, once obliteration was achieved, no variceal bleeding episodes were observed. The frequency of mild complications associated with EVBL therapy using multiple ligators was reasonable and consistent with other studies. Superficial ulcers or their residuals were uniformly seen (conditio sine qua non). However, deep ligation-induced ulcers leading to bleeding were observed in two patients and resulted in death in one of them. The variceal recurrence rate of 11% was very low, but similar to that reported by Jutabha, Sarin and Lui.1520-21 In contrast, Schepke, et al. reported a recurrence rate of 60% over 34.4 ± 18.9 months of follow-up.16 It is possible that this difference resulted from either the shorter follow-up period or to a higher proportion of patient with relatively well-preserved liver functions (93.2 % Child A and B in our study). These interesting findings warrant further investigation.
Pereira-Lima, et al. reported that PHG worsened in 42.8% of patients with variceal bleeding treated with band ligation.26 In contrast, only 12.5% of patients in our study experienced deterioration of PHG. PHG even improved in 33.3% of patients following prophylactic EVBL. This may be explained by a more advanced stage of portal hypertension, as well as liver cirrhosis in patients who present with variceal bleeding as compared to those undergoing prophylactic treatment and have never bled.
At present β-blockers are the only drugs recommended for primary prophylaxis of first variceal hemorrhage. Despite their obvious advantages, such as low cost and easy administration, they fail to work in some patients and are associated with a high occurrence of side effects. The frequency of side effects in our study population was high (44%), however they were mild, transient and did not result in treatment discontinuation in any patient. Adequate reduction of pulse rate was not achieved in 12% of patients. The mean propranolol dose required in our patients to achieve response was quite low and similar to that previously reported.16,20
The frequency of mild side effects was comparable in the two groups. However, the frequency of severe side effects was higher in the EVBL group, though this difference was not statistically significant. Deep post-ligation ulcers caused bleeding in two patients and resulted in death in one of them. The reason for this complication may be, in our opinion, related to a suboptimal procedural technique. Similar findings have been reported by others.23 Thus, this risk needs to be taken into consideration, especially in the setting of a prophylactic treatment and providing that there were no serious side effects in the propranolol group.
The actuarial probabilities of mortality in our study in the EVBL and propranolol groups were lower than previously reported (7% and 5%, respectively).15-17,20 This may be explained by the fact that more than 50% of patients enrolled in our study had compensated liver cirrhosis. The actuarial bleeding rate after 18 months of 20% in both groups was comparable with most previous studies. The first published comparative study of EVL and propranolol showed a very high actuarial bleeding rate after 18 months in the propranolol arm (43%), but the degree of compliance was questionable.20 A more recent trial and meta-analysis reported 2-year actuarial bleeding rates of 17.7% and 22%, respectively.7,16
Our study has several shortcomings. First, the follow-up period may not have been adequate to provide a definitive conclusion. We cannot rule out the possibility that the variceal recurrence rate would have been greater in the EVBL group or patients’compliance and propranolol effect lower in the b-blocker group over a longer period of time. Nevertheless, in the very few published studies with longer follow-up periods, the majority of bleeds or deaths occurred in the first 18 months.16,17 Second, although the sample size calculation was based on previously reported results, interim analysis showed that the number of patients enrolled in the study did not provide sufficient power to detect a significant difference between treatments. However, due to the rarity of this clinical entity and small differences between the study groups, enrolling large numbers of patients has proven difficult even in a multicenter setting. Other published studies have been interrupted for similar reasons.16,17
However, studies such as ours can be used in subsequent meta-analyses. To show the importance and usefulness of our study, we analyzed our data together with data from a recent similar study of Perez-Ayuso that surprisingly showed almost a five times higher bleeding rate from esophageal varices in the propranolol group. The meta-analysis revealed several points of agreement between the two studies including no difference in mortality and overall bleeding. Nevertheless, the difference in esophageal variceal bleeding observed in the study by Perez-Ayuso was no longer significant. Thus, increasing the number of patients may lead not only to increasing the power of a negative result, but also to overrule a false positive finding that may have resulted from a small sample size.
In conclusion, for patients with liver cirrhosis large esophageal varices and no history of varicea hemorrhage, both EVL and propranolol were equa lly effective and safe. The prophylactic EVBL hac some clinically significant complications, but eradi cation was achieved rapidly. Moreover, once oblite ration was achieved, no subsequent varicea bleeding episodes were observed. Thus, EVBL can be offered as a safe and effective alternative in patients with low compliance or absent hemodynamic respon se to propranolol therapy, although its potentia risk of significant complications must be considered.
Abbreviations- •
HVPG: Hepatic venous wedge pressure gradient.
- •
EVBL: Endoscopic variceal band ligation.
- •
PHG: Portal hypertensive gastropathy.
We would like to thank V. Lanska from Institute for Clinical and Experimental Medicine (Prague Czech Republic) for help with the statistica analysis.