Objective. There are evidences that the changes in glycosylation and sialylation of proteins and lipids play an important role in the pathogenesis and progression of various liver diseases. The aim of this study was to evaluate the changes in the sialylation of serum lipids measured by the level of lipid-bound sialic acid (LSA) in liver diseases of different etiologies.
Materials and methods. Tested group consisted of 303 patients suffering from liver diseases: alcoholic and non-alcoholic cirrhosis, chronic non-viral hepatitis, toxic hepatitis, chronic viral C and B hepatitis, autoimmune hepatitis, primary liver cancer, liver cancer and cirrhosis (mixed group), acute hepatitis B, primary biliary cirrhosis and fatty liver. LSA was determined by the method of Katopodis and co-workers.
Results. There were significant differences in the serum LSA concentrations between liver diseases of different etiologies. The level of LSA in liver tumors was higher than that in both types of cirrhosis: alcoholic and non-alcoholic. In turn, LSA level in non-alcoholic cirrhosis was lower than in toxic hepatitis and mixed group. There was no difference in LSA concentration between tumor and mixed group. Similarly to LSA, AFP level in tumor group was also higher than that in both cirrhotic groups, but there was no difference in AFP concentration between tumor and mixed group.
Conclusions. The sialylation of serum lipids alters in liver diseases of different etiologies. Given the importance of glycans in biological systems we can speculate that the changes in lipids sialylation play an important role in liver pathology, especially in primary cancer, cirrhosis and toxic hepatitis.
The terminal position in carbohydrate chains of proteins and lipids constituents of plasma lipoproteins is occupied by sialic acid (SA).1 This location predisposes its to the participation in cellular and molecular interactions and this way sialic acid plays a significant role in lipoproteins and lipids metabolism.2,3 Glycosylation and sialylation of lipids and proteins takes place in the liver. There are evidences that the changes in glycosylation or sialylation of proteins and lipids have an important role in the pathogenesis and progression of various liver diseases.4,5
The group of sialic acid-containing constituents of lipoproteins are glycolipids.6 The most characteristic of their representative in the blood are gangliosides.7 In liver diseases of different etiologies, the concentration, pattern and distribution of gangliosides in lipoproteins are altered.8 The content of sialic acid associated with lipid part of lipoproteins can be determined as a lipid-bound sialic acid (LSA).9 The aim of this study was to evaluate the changes in the sialylation of lipids measured by the level of lipid-bound sialic acid in liver diseases of different etiologies (chronic hepatitis, liver cirrhosis, primary biliary cirrhosis, toxic hepatitis, primary liver cancers, fatty liver, autoimmune hepatitis and acute hepatitis B).
Experimental ProceduresSubjectsThis study was approved ethically by the Bioethical Committee working at the Medical University in Bialystok (Approval No. R-I-002/192/2009). All patients provided informed written consent.
The tested group consisted of 303 consecutive patients (108 females and 195 males) (mean age: 55 years; range: 20-84) who were admitted to the Department of Infectious Diseases. The patients were divided into subgroups according to the diagnosis of liver diseases: 56 had alcoholic cirrhosis (AC), 35 non-alcoholic cirrhosis (NAC), 25 chronic non-viral hepatitis (CH), 34 toxic hepatitis (TH), 24 chronic viral hepatitis C (HCV), 20 chronic viral hepatitis B (HBV), 15 autoimmune hepatitis (AIH), 26 primary liver cancer (HCC), 21 primary liver cancer and cirrhosis (mixed group, HCC +C), 16 acute hepatitis B (AHB), 17 primary biliary cirrhosis (PBC), 14 fatty liver (FL). The diagnosis was performed on the basis of signs and symptoms of the disease, physical and clinical exam (abdominal ultrasound, liver biopsy in selected cases), and biochemical liver panel known as liver function tests which included alanine and aspartate aminotransferase (ALT and AST), γ-glutamyl transferase (γ-GT), bilirubin, albumin and total protein. The serological tests (HBsAg, anti-HCV) were also used to support the diagnosis of viral infections. Non-alcoholic liver cirrhosis was in decompensated stage in 20 patients. The causes of this disease were in 14 patients - infections of HBV, in 9 patients-infections of HCV, in the rest patients - unidentified factors. Toxic hepatitis was most frequently caused by acute alcohol abuse (24 patients) and drugs abuse (10 patients). To confirm the diagnosis of primary biliary cirrhosis the mitochondrial antibody test (AMA test) was performed.
The control group consisted of 80 healthy subjects recruited from hospital workers (mean age: 55 years; range: 22-77; 36 females and 44 males). All subjects (healthy and sick) gave their consent to participate in the studies.
Blood samples were taken by vein puncture once after admittance before treatment. The blood was allowed to clot. The sera were separated by centrifugation at 1,500 × g for 10 min at room temperature and stored at-86 °C until analysis.
Laboratory assaysSerum LSA concentration was measured according to the method described by Katopodis and Stock.9 All reagents were delivered by Sigma-Aldrich Chemie GmbH (Steinheim, Germany). Briefly, 50 μL of serum was diluted with 150 μL of distilled water, capped, vortexed for 10 seconds, and extracted for 30 seconds with 3 mL of cold (4 °C) a chloroform-methanol mixture (2:1 v/v). To this mixture was added 0.5 mL of cold distilled water, and the tubes were recapped, and mixed again for 30 seconds by repeated inversion. The tubes were centrifuged for 5 minutes at 2500 rpm at room temperature. After then, 1 mL of the upper layer was transferred to another tube and was treated with 50 μL of a phosphotungstic acid solution (1 g/mL). The tubes were vortexed and allowed to sit at room temperature for 5 minutes. After centrifugation for 5 minutes at 2500 rpm the supernatant was removed by suction. The remaining pellets was redissolved in 1 mL of water (37 °C) by vigorously vortexing for 1 minute. For determination of LSA, to each tube was added 1 mL of resorcinol reagent (including 10 ml of 2% (w/v) resorcinol in water, 9.75 mL water, 0.25 mL of 0.1 M CuSO4, brought to a final volume of 100 mL with concentrated HCl). The tubes were mixed and placed in boiling water for 15 minutes. Immediately after that, the tubes were transferred to an ice bath and left for 10 minutes. To each tube 2 mL of butylacetate/n-butanol (85:15 v/v) was added, the tubes were vortexed and centrifuged at 2500 rpm for 10 minutes at room temperature. The blue color was read at 580 nm on the spectrophotometer (Shimadzu UV-1202, Shimadzu Europa GmbH, Duisburg, Germany). The amount of LSA was read from standard curve (5, 10, 20 and 30 μg/mL of solutions of N-acetylneuraminic acid prepared from 40 μg/mL stock solution). The concentration of LSA was calculated using the following formula:
LSA (mg/dL) = A × 100,000 μL/B × 45 μL × 1,000
A-μg of N-acetylneuraminic acid (NANA) read from standard curve.
B-1.0/1.3 a factor to correct of the volume in the extraction step.
Alpha-fetoprotein (AFP) concentration in the sera of cancer and cirrhotic patients was determined in the chemiluminescent microparticle immunoassay test (CMIA) (Abbott Ireland, Diagnostics Division, Finisklin Business Park, Sligo, Ireland) on the Architect i2000 system (Abbott Laboratories, Abbott Park, ILL., USA). In this test, the AFP concentrations in the 400 healthy subjects ranged from 1.09 to 8.04 ng/mL (95% observations).
StatisticsTo tested the effect of liver diseases on the concentration of LSA, ANOVA rank Kruskal-Wallis test was performed. Because the chance of finding one or more significant differences in 12 tested groups was 45.96% (Bonferroni correction factor), we performed the non-parametric multiple comparison test (post-hoc test for Kruskal-Wallis) to ascertain which the intermediate medians are significantly different. The differences between tested and control groups were evaluated by Mann-Whitney U test. We considered P values less than 0.05 as statistically significant.
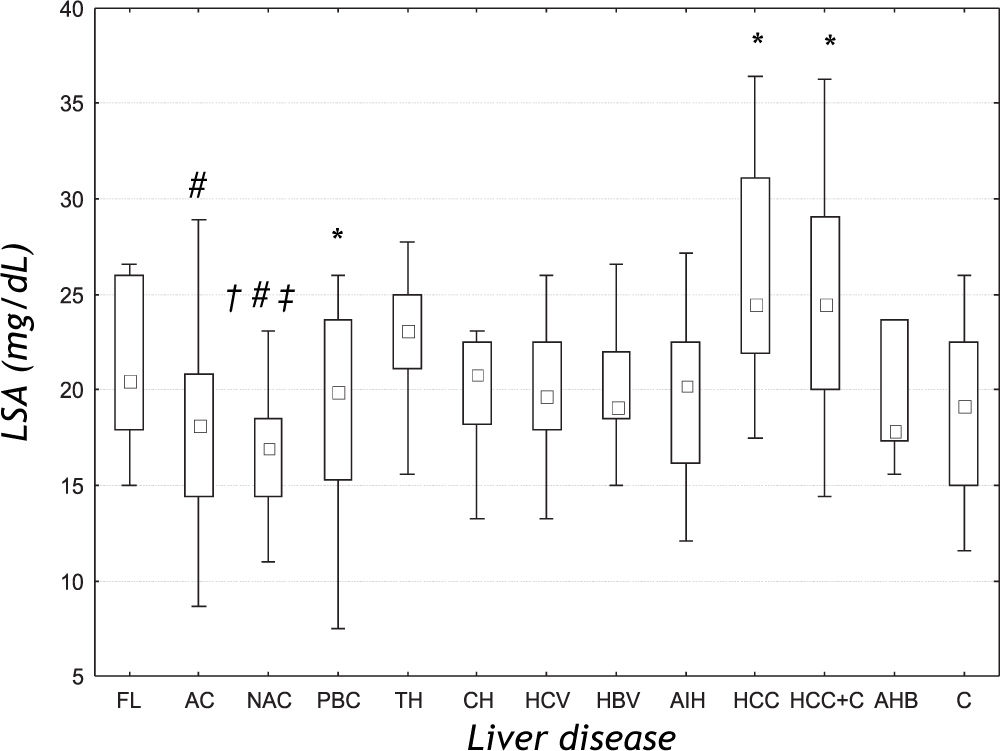
ResultsThere were significant differences in the serum LSA concentrations between liver diseases of different etiologies (ANOVA rank Kruskal-Wallis test: H = 43.178, P < 0.001) (Figure 1). Further analysis revealed that the mean LSA concentration in cancer group (HCC), cancer and cirrhosis group (HCC+C) and toxic hepatitis group (TH) was significantly increased when compared with the control group (P = 0.001; P = 0.021 and P = 0.017, respectively). Post-hoc analysis for Kruskal-Wallis test revealed that the median LSA value for the cancer group (HCC) was significantly higher than the mean value for alcoholic (AC) (P < 0.001) and non-alcoholic cirrhosis (NAC) (P < 0.001). Additionally, the mean LSA values for non-alcoholic cirrhosis (NAC) were significantly lower than the mean values for toxic hepatitis group (TH) (P = 0.006) and for mixed group (HCC+C) (p = 0.029). There was no significant difference between tumor (HCC) and mixed group (HCC+C) (P = 0.638). In the primary biliary cirrhosis (PBC), the mean LSA value was not significantly different from the mean values for other liver diseases.
LSA concentrations in the sera of patients with liver diseases of different etiologies. Results are presented as median and range. FL: Fatty liver. AC: Alcoholic cirrhosis. NAC: Non-alcoholic cirrhosis. PBC: Primary biliary cirrhosis. TH: Toxic hepatitis. CH: Chronic non-viral hepatitis. HCV: Chronic viral C hepatitis. HBV: Chronic viral B hepatitis. AIH: Autoimmune hepatitis. HCC: Primary liver cancer. HCC+C: Primary liver cancer and cirrhosis. AHB: Acute viral B hepatitis. C: Control group. # P < 0.05 compared with HCC group. * P < 0.05 compared with C group. † P < 0.05 compared with AC group. ‡ P<0.05 compared with TH group.
The analysis of AFP concentration in cirrhotic (alcoholic and non-alcoholic) and cancer patients (tumor and mixed group) revealed that mean AFP level significantly differ between these diseases (H = 23.148, P < 0.001). Post-hoc analysis revealed that the median in tumor group (39.85 ng/mL; range: 5.94-92.20) was higher than that in alcoholic cirrhotic group (5.14 ng/mL; range: 2.04-23.40) and nonalcoholic cirrhotic group (5.80 ng/mL; range: 1.64-37.57) (P = 0.008 and P = 0.006, respectively). Median for mixed group (19.95 ng/mL; range: 3.6383.42) was also higher than the medians for both cirrhotic groups (P = 0.003 and P = 0.002, respectively for alcoholic and non-alcoholic cirrhosis). There was no difference in AFP concentration between tumor and mixed group (P = 1.000).
DiscussionIncreased levels of serum sialic acid (total and lipid-bound) have been observed in different conditions, especially in malignancies, inflammatory disorders, cardiovascular diseases, diabetes, alcohol abuse, inherited disorders of sialic acid as well as acute-phase reactions.4,10 Most recently the variations of serum total sialic acid (TSA) level in liver cirrhosis, fatty liver, liver cancer and acute and chronic hepatitis have been described.11 Because the synthesis (sialylation too), degradation (also desialylation) and storage the lipids and lipoproteins are attributed to the liver, we can suspect that the liver diseases affect not only the serum level of lipids and lipoproteins, but also the level of sialic acid bounded with these compounds, that means LSA. In our study, the serum level of LSA appears to be different in liver diseases. We denoted higher level of LSA in primary liver cancers than in liver cirrhosis of both origin (alcoholic and non-alcoholic), and also the higher LSA concentration in toxic hepatitis than in non-alcoholic cirrhosis. The levels in cancer group, cancer and cirrhosis group, and toxic hepatitis were higher than that in the controls. Matsuzaki, et al.12 found higher TSA level in hepatoma and metastatic liver cancer than in the control subjects and also higher than in the cirrhosis group. Increased concentration of LSA in malignancies can be explained by the alterations in the metabolism of tumor cell surface glycoproteins and sialoglycolipids owing the activation of tumor characteristic enzymes-glycosyl transferases.13 The sialic acid enriched compounds of tumor cell surface are sheded or secreted by these cells to the blood, contributing to the increase of sialic acid concentration which predominantly is bounded to lipids. However, this mechanism could explain the elevated levels of LSA in liver cancers, but not in diseases of other etiologies. It should be emphasized, that LSA level differs between liver tumors and cirrhosis, similarly to AFP concentration. However, neither of them does not differ between alcoholic and non-alcoholic cirrhosis or between liver tumors and tumors with cirrhosis.
Characteristic pattern of LSA in our study consist also in increased level in toxic hepatitis, which was additionally higher than that in non-alcoholic cirrhosis, and higher in tumor and cirrhosis group in comparison to non-alcoholic cirrhosis. In general, LSA levels in both types of cirrhosis were the lowest among the compared liver diseases. The explanation for the increased LSA concentration in toxic hepatitis and the decreased level in cirrhosis may be the changes in the sialyltransferase activity during acute and chronic stress.14 Acute stress resulted in increase of this enzyme activity in liver and spleen, but the chronic stress resulted in decrease of enzyme activity in liver. Stefenelli, et al,15 denoted that also the levels of TSA in chronic liver diseases, including cirrhosis, were lower than that in the control samples and differed from other tested groups (pneumonia, rheumatoid arthritis and other inflammatory diseases). From the other hand, the concentrations of LSA in hepatitis of different etiologies (chronic and acute viral, chronic non-viral, toxic and autoimmune) and fatty liver do not differ. Similarly, the results of TSA in chronic and acute hepatitis, and in fatty liver resemble each other.12 Thus, there were differences in serum LSA concentrations between liver diseases, especially between non-alcoholic liver cirrhosis and toxic hepatitis. It is also interesting, that there is no the difference between alcoholic and non-alcoholic cirrhosis. In our previously published study, the similar results of TSA in alcoholic and non-alcoholic cirrhosis were obtained.16
ConclusionOur findings show that the LSA level differs significantly between liver diseases, especially between primary liver tumors, cirrhosis and toxic hepatitis. The changed concentrations of serum lipid-bound sialic acid in liver diseases are the result the alteration in sialylation of lipids parts of lipoproteins (especially glycolipids) and can affect the pathogenesis and progression of various liver diseases.
Abbreviations- •
SA: Sialic acid.
- •
LSA: Lipid-bound sialic acid.
- •
AC: Alcoholic cirrhosis.
- •
NAC: Non-alcoholic cirrhosis.
- •
CH: Chronic non-viral hepatitis.
- •
TH: Toxic hepatitis.
- •
HCV: Chronic viral hepatitis C.
- •
HBV: Chronic viral hepatitis B.
- •
AIH: Autoimmune hepatitis.
- •
HCC: Primary liver cancer.
- •
HCC+C: Primary liver cancer and cirrhosis.
- •
AHB: Acute hepatitis B.
- •
PBC: Primary biliary cirrhosis.
- •
FL: Fatty liver.
- •
ALT: Alanine aminotransferase.
- •
AST: Aspartate aminotransferase.
- •
GGT: γ-glutamyl transferase.
- •
AMA: Mitochondrial antibodies.
- •
NANA: N-acetylneuraminic acid.
- •
TSA: Total sialic acid.