Chronic hepatitis C virus (HCV) infection and autoimmune disorders show a complex interplay, with HCV often being identified as the trigger of autoimmune phenomena or diseases. While there is evidence of successful HCV treatment with direct-acting antivirals (DAA) in patients with concomitant HCV and autoimmune hepatitis (AIH), there are also sparse reports of AIH developing during, or following, DAA treatment.
Here we report a case of a patient with suspected concomitant HCV and AIH who underwent liver biopsy but showed no histological hallmarks of autoimmunity. The patient later developed a hepatitic flare following DAA-induced viral clearance, and a second liver biopsy showed features compatible with AIH. Response to corticosteroid and azathioprine treatment was seen. This reports demonstrates that patients with features of auto-reactivity and HCV after DAA-induced viral clearance require careful follow-up.
Chronic infection with the hepatitis C virus (HCV) and autoimmune disorders show a complex interplay, and HCV may be identified as the trigger of autoimmune phenomena or diseases in some patients. While there is evidence of successful HCV treatment with direct-acting antivirals (DAA) in patients with concomitant HCV and autoimmune hepatitis (AIH), there are also sparse reports of the development of AIH during, or following, DAA treatment. Indeed, some cases of treatment-related emergence of AIH have been reported following HCV RNA clearance in HCV patients treated with DAAs, although in some of these cases AIH developed in patients with a history of autoimmune diseases [1–4].
Smooth muscle antibodies (SMA) and anti-nuclear antibodies (ANA) can be found in the sera of patients with chronic HCV infection, although they do not seem to have an impact on either biochemical or histological features [4–7]. These autoantibodies are also the immunological hallmark of type I AIH, a disease whose diagnosis may be challenging especially when other potential causes of liver disease, such as chronic HCV infection, are concomitant. In these cases, while liver biopsy may help the clinician, the distinction between HCV- and AIH-associated inflammation at histology can be difficult even for experienced pathologists [1,8,9].
Here we describe a gentleman with chronic HCV who had positivity for ANA and SMA, and elevated immunoglobulin: despite the first liver biopsy not showing definite features of autoimmunity, the patient presented an aminotransferase flare following DAA-induced viral clearance. A second liver biopsy, performed approximately one year after SVR, was indeed compatible with a diagnosis of AIH that responded to immunosuppressive treatment.
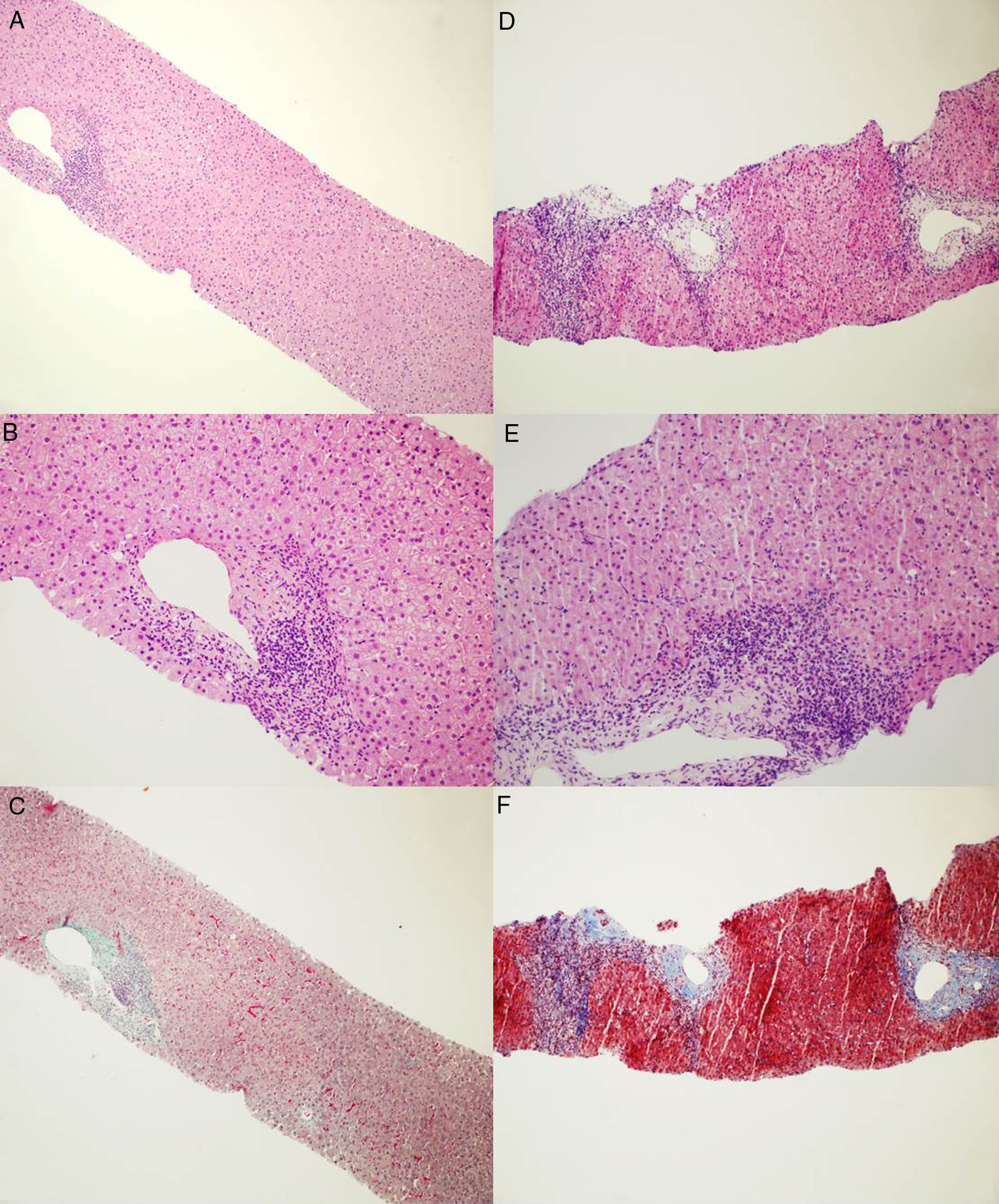
2Case reportA 66-year old gentleman was referred to our outpatient clinic due to untreated HCV infection (genotype 2) for 18 years. Medical history included appendectomy, bilateral inguinal hernia repair and neurosurgery for spinal disc herniation. At referral, blood tests showed elevated alanine aminotransferase level [alanine aminotransferase (ALT) 4 times the upper limit of normal (ULN)], elevated immunoglobulin (20.8%), ANA (>1:320) and SMA (1:160) positivity. Transient elastography showed absence of liver fibrosis (liver stiffness, 4.5kPa), and abdominal ultrasound was unremarkable. Due to the discrepancy between non-invasive staging and the bio-humoral picture (elevated immunoglobulins, ANA and SMA positivity) a suspicion of concurrent AIH was raised and the patient underwent liver biopsy. Liver histology (21mm biopsy; 12 complete portal tracts) showed mild portal tract lymphocyte-predominant inflammation with few small lymphoid aggregates, minimal piecemeal necrosis and fibrous expansion of few portal areas without septa (Metavir F1) (Fig. 1A), findings were consistent with mild chronic hepatitis compatible with HCV infection, without histological stigmata of autoimmunity [8]. The AIH score was 9, and the simplified AIH score was 4 [8,10].
Microphotographs of liver histology. (A and B) First liver biopsy showing mild inflammation within portal tracts with lymphoid aggregate and mild interface activity consistent with HCV infection. No clear morphological evidence of autoimmunity. [Haematoxylin and Eosin stain. Magnification ×100 for A and ×200 for B]. (C) First liver biopsy stained for collagen showing a mild increase in portal fibrosis without evidence of fibrous septa or fibrous bridge formation. [Masson's Trichrome stain. Magnification ×100]. (D and E) Second liver biopsy showing moderate-severe portal inflammation with marked interface activity and moderate lobular inflammation with plasmacells. [Haematoxylin and Eosin stain. Magnification ×100 for D and ×200 for E]. (F) Second liver biopsy stained for collagen showing increased fibrosis with portal expansion and porto-portal fibrous septa at least. [Azan Mallory's Trichrome stain. Magnification ×100].
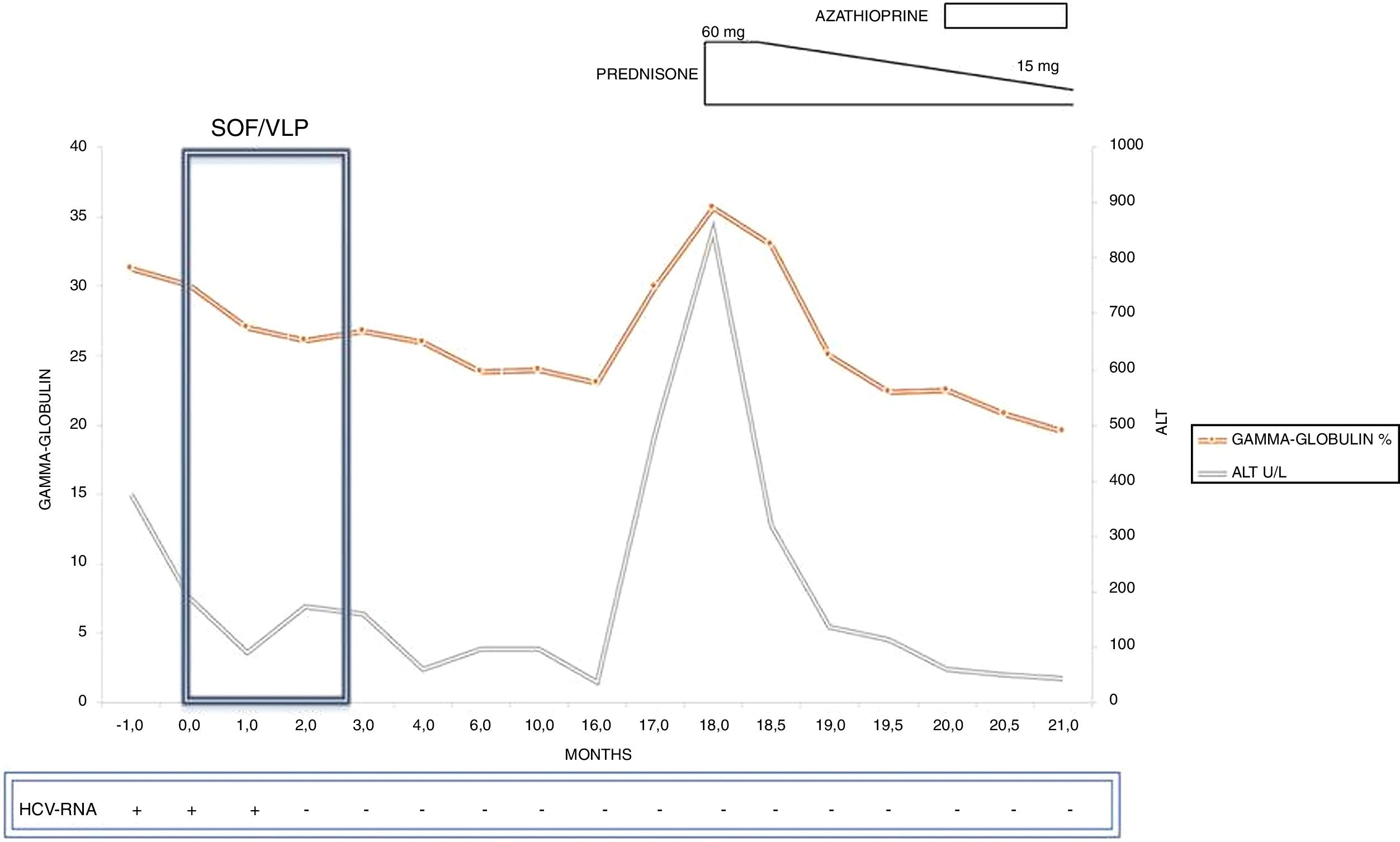
A 12-week course of sofosbuvir 400mg/velpatasvir 100mg daily was started. Before the start of DAA therapy, liver biochemistry revealed a further increase in ALT (9 times ULN) and gammaglobulins (31.2%). The patient completed the DAA course uneventfully, achieving week-12 sustained virological response (SVR). ALT and gammaglobulin levels however remained persistently above the ULN during both treatment and follow-up (Fig. 2). No other causes of liver damage (concurrent hepatitis B virus infection, alcohol, metabolic syndrome, drugs or over-the-counter medications or herbal remedies) were identified.
The patient was monitored every 3 months following SVR, and during this period ALT and gammaglobulin never normalized while approximately one year after SVR an ALT flare (>20 times ULN) was observed together with a further increase in gammaglobulin levels (35.7%). Due to these findings, in consideration of the patient's autoimmunity profile, and the apparent progression of liver stiffness at transient elastography (liver stiffness, 7.4Kpa), a second liver biopsy was performed. Histology (three cores: 11, 9, and 7mm; 15 complete portal tracts) showed moderate to marked portal inflammation with lymphocytes and plasma-cells, severe interface hepatitis, and portal tract fibrosis with formation of initial porto-portal septa (Metavir F2) (Fig. 1B), consistent with an overall picture of moderate-to-severe chronic hepatitis and moderate liver fibrosis, with histopathological hallmarks of autoimmunity-induced damage (pre-treatment AIH score, 18; simplified AIH score, 8). Hence, the patient was initially treated with a tapering-dose prednisolone regimen and, after a significant decrease in ALT, azathioprine (2mg/kg body weight) was commenced, with a prompt and marked improvement in liver biochemistry (AST 37U/L; ALT 42U/L; gammaglobulin 19.5%). The patient is currently being followed-up on azathioprine maintenance treatment, with normal aminotransferase and normal gammaglobulin levels (post-treatment AIH score, 20).
3DiscussionThe case reported here is emblematic of the potential, dynamic interplay between HCV infection and autoimmunity: the patient had a pre-treatment clinical suspicion of coexistent AIH and HCV infection (ANA and SMA positivity, elevated gammaglobulins despite the presence of low liver stiffness values) although liver biopsy – adequate for interpretation and read by an expert liver pathologist – showed no features suggestive of AIH,. Both complete and simplified AIH scores were not indicative of AIH [8,10]. However, the absence of ALT and gammaglobulin normalization, as is commonly observed following successful DAA-induced HCV clearance, and, in particular, a paradoxical ALT flare in the course of follow-up prompted liver biopsy repetition whichshowed histological findings associated with AIH. AIH score totaled 18 points [8,11,12]. The final diagnosis was corroborated by the absence of other causes of liver disease and the positive response to immunosuppressive treatment (post-treatment AIH score, 20).
In this case, co-existence of viral infection and immune-activation with features of hepatic autoimmunity were probably already present even before antiviral therapy-A possible hypothesis is that immunity mechanisms might have been more engaged in the fight against HCV rather than in inducing a true autoimmune-mediated liver injury, and that following resolution of viral infection the previously smouldering hepatic autoimmunity might have flourished, as one of its targets had been cleared. These hypotheses are supported by the presence of serological markers of autoimmunity since diagnosis, despite non-suggestive liver histology, and by the clinical course of disease after viral clearance, coupled with a dramatic modification in hepatic histopathology. However, as the liver disorder worsened after DAA treatment, one cannot exclude that AIH may actually have been induced by antiviral treatment, through potential mechanisms that have not been precisely identified and that were recently reviewed by Kanda et al. [12].
We feel that our findings are relevant to the management of patients with HCV and a strong suspicion of AIH. In the past the link between HCV and autoimmunity has been a reason for deferring interferon-based therapy due to the risk of exacerbating a latent autoimmune disorder. This barrier has recently been lifted following the advent of DAAs, which offer the opportunity to safely treat HCV patients with features of autoimmunity. In fact, while on the one hand DAA-induced viral clearance may be associated with disappearance of severe immune-mediated diseases, and cirrhotic patients with concomitant AIH and HCV may greatly benefit from viral clearance, on the other hand there is evidence of potential worsening of underlying AIH and autoimmune phenomena following successful DAA treatment of HCV, as well as reports of treatment-related emergence of AIH following DAA-induced SVR [1,2,13–16]. Lastly, there is also evidence, provided by recent studies, that successful clearance of HCV with DAA therapy is associated with the complete resolution of serum markers of autoimmunity in approximately half of the population who had these characteristics before treatment, while in a cohort of HCV patients with concomitant AIH and HCV infection, at least one patient had an exacerbation of AIH and of a coexistent autoimmune disease (ulcerative colitis) following DAA treatment (although unfortunately liver histology was not obtained at hepatitis flare) [4,17].
Our suggestion, on the basis of the case reported here and on the accumulating evidence in the literature, is that patients with HCV hepatitis and features suggestive of hepatic autoimmunity should be accurately evaluated before treatment, and closely monitored after successful DAA treatment. A prolonging of the usually short-term biochemical evaluation that follows SVR is advocated as, in some instances, the initial diagnosis may need to be re-evaluated, with potential reflection on treatment and prognosis.AbbreviationsALT
alanine aminotransferase
ANAanti-nuclear antibodies
AIHautoimmune hepatitis
DAAdirect-acting antivirals
HCVhepatitis C virus
SMAsmooth muscle antibodies
SVRsustained virological response
ULNupper limit of normal
Authors’ contributionDrafting of the manuscript: VC, EC, EGG; critical revision of the manuscript for important intellectual content: GB, MF, EM, FG, and EGG; performing histological analysis: FG; study supervision: EGG.
Informed consentInformed patient consent was obtained for publication of the case details.
FundingNo financial support.
Conflict of interestThe authors have no conflicts of interest to declare.





![Microphotographs of liver histology. (A and B) First liver biopsy showing mild inflammation within portal tracts with lymphoid aggregate and mild interface activity consistent with HCV infection. No clear morphological evidence of autoimmunity. [Haematoxylin and Eosin stain. Magnification ×100 for A and ×200 for B]. (C) First liver biopsy stained for collagen showing a mild increase in portal fibrosis without evidence of fibrous septa or fibrous bridge formation. [Masson Microphotographs of liver histology. (A and B) First liver biopsy showing mild inflammation within portal tracts with lymphoid aggregate and mild interface activity consistent with HCV infection. No clear morphological evidence of autoimmunity. [Haematoxylin and Eosin stain. Magnification ×100 for A and ×200 for B]. (C) First liver biopsy stained for collagen showing a mild increase in portal fibrosis without evidence of fibrous septa or fibrous bridge formation. [Masson](https://static.elsevier.es/multimedia/16652681/0000001900000002/v1_202003030740/S166526811932277X/v1_202003030740/en/main.assets/thumbnail/gr1.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)





