Some studies have pointed to a role of TNF-alpha in pathogenesis of non alcoholic fatty liver disease (NAFLD). The aim of our study was to investigate the influence of G308A polymorphism TNF alpha gene on the histological changes, insulin resistance and TNF-alpha levels in overweight patients. A population of 66 patients with NAFLD was recruited in a cross sectional study. A biochemical analysis of serum was measured. Genotype of TNF alpha gene G308A was studied. Fifteen patients (22.7%) had the genotype G308A (mutant type group) and 51 patients (77.3%) G308G (wild type group). Patients with mutant type group presented more moderate-severe portal inflammation (86.7%) in liver biopsy compared to patient with wild genotype (19.7%). Mutant type group had more moderate-severe fibrosis (73.3%) than wild type group (51.3%). The multivariate analysis adjusted by age, sex, BMI and genotype with the dependent variable (fibrosis) showed that HOMA remained in the model, with an increase of the probability to develop fibrosis of 1.78 (CI95%:1.06-3.2) and develop moderate-severe inflammation of 1.45 (CI95%:1.02-2.1) with each increase of one unit on HOMA levels. In conclusion, Patients with mutant genotype have more frequently moderate-severe portal inflammation and fibrosis than wild type genotype.
Non-alcoholic fatty liver disease (NAFLD) is a liver disease characterized by elevated serum amino-transferase levels, hepatomegaly and accumulation of fat in liver accompanied by inflammation and necrosis resembling alcoholic hepatitis in the absence of heavy alcohol consumption.1
Obesity is considered the most important risk factor. In different studies, waist to hip circumference ratio was correlated with degree of steatosis on liver biopsy.2 Insulin resistance has been associated with fat liver and NAFLD, too.3 The association with insulin resistance and obesity has also suggested that NAFLD should be considered part of the metabolic syndrome.4
Adipose tissue secretes several bioactive proteins, or adipocytokines, that regulate hepatic and peripheral glucose and lipid metabolism. These adipocytokines include leptin, resistin, adiponec-tin and tumor necrosis factor alpha (TNF-alpha). Recently, Hui et al.5 suggested that raised serum leptin levels in non alcoholic steatohepatitis (NASH) may be a reflection of the failure of leptin to stimulate hepatic lipid turnover, it could be called as a leptin resistance status. Some evidences have linked tumor necrosis factor alpha (TNF alpha) to the metabolic abnormalities of insulin resistance and adipose tissue has been shown to be a site for TNF-alpha synthesis, with a direct correlation between obesity, TNF-alpha levels and hyperinsulinemia.6 TNF-alpha is one of the most important adipocytokines, however the role of this molecule on NFALD pathogenesis has not been explored and it relationship with genetic factors such as single nucleotide polymorphism remains unknown.
Mutation analysis has identified a G-> A transition in the promoter region of TNF-alpha gene at position-308. This polymorphic variant has been shown to affect the promoter region of the TNF-al-pha gene leading to a higher rate of transcription compared to the wild allele.7 Several association studies have been conducted on the G-308 variant, with conflicting results. Fernandez Real, et al.8 has reported a significant association between the G-308A variant and increased BMI and insulin resistance.
The aim of our study was to investigate the relationship of G308A polymorphism TNF alpha gene on the liver histological changes, insulin resistance and TNF-alpha levels in overweight patients.
Subjects And MethodsSubjectsConsecutive 66 Caucasian overweight subjects seen between 2006 and 2009 were enrolled for this study. The exclusion criterias were hepatitis B, C, cytomegalovirus, Epstein Barr infections, nonorgan-specific autoantibodies, alcohol consumption, diabetes mellitus, fasting glucose intolerance, medication (blood-pressure lowering medication and statins) and hereditary defects (iron and copper storage diseases and alpha 1-antitrypsin deficiency). A control group of 213 obese patients were recruited to compare the distribution of 308 genotype between NA-FLD and control subjects. The study was approved by the institutional ethics committee. These patients were studied in a Nutrition Clinic Unit and signed an informed consent.
Liver biopsiesThe diagnosis of NAFLD was confirmed by percutaneous liver biopsy performed in all subjects with a 1.6 mm Menghini-type biopsy needle. Liver samples were routinely processed, sectioned, and stained with hematoxilin-eosin and Manson’s trichome. All biopsies were studied by the same liver pathologist (T.A.G.). Histology was analysed using a modified version of the Brunt classification.9 Steatosis was graded as follows: mild (< 33% of hepatocytes affected); moderate-severe (≥ 33% of hepatocytes affected). The Brunt system also includes as grading: portal inflammation, balloning, lobular inflammation and stating fibrosis:
- •
Stage 1: Zone 3 perivenular perisinusoidal/peri-cellular fibrosis, focal or extensive;
- •
Stage 2: As above with focal or extensive peri-portal fibrosis;
- •
Stage 3: Bridging fibrosis, focal or extensive;
- •
Stage 4: Cirrhosis.
In our study, fibrosis variable was graded as absent or presence and inflammation (portal and lobular) stage was divided as mild or moderate-severe.
Anthropometric measurementsBody weight was measured to an accuracy of 0.5 kg and body mass index computed as body weight/ (height2). Waist (narrowest diameter between xiphoid process and iliac crest) and hip (widest diameter over greater trochanters) circumferences to derive waist-to hip ratio (WHR) were measured, too. Tetrapolar body electrical bioimpedance was used to determine body composition. An electric current of 0.8 mA and 50 kHz was produced by a calibrated signal generator (Biodynamics Model 310e, Seattle, WA, USA) and applied to the skin using adhesive electrodes placed on right-side limbs. Resistance and reactance were used to calculate total body water, fat and fat-free mass.
ProceduresBasal glucose, insulin, insulin resistance (HOMA), total cholesterol, LDL-cholesterol, HDL-cholesterol, triglycerides and TNF-alpha blood le-vels were analyzed in fasting samples.
Serum total cholesterol and triglyceride concentrations were determined by enzymatic colorimetric assay (Technicon Instruments, Ltd., New York, N.Y., USA), while HDL cholesterol was determined enzymatically in the supernatant after precipitation of other lipoproteins with dextran sulfate-mag-nesium. LDL cholesterol was calculated using Frie-dewald formula.
Plasma glucose levels were determined by using an automated glucose oxidase method (Glucose analyser 2, Beckman Instruments, Fullerton, California). Insulin was measured by RIA (RIA Diagnostic Corporation, Los Angeles, CA) with a sensitivity of 0.5 mUI/L (normal range 0.5-30 mUI/L) (10) and the homeostasis model assessment for insulin sensitivity (HOMA) was calculated using these values.11
TNF alpha was measured by ELISA (R&D systems, Inc., Mineapolis, USA) with a sensitivity of 0.7 pg/mL. Normal values of TNF alpha was (0.515.6 pg/mL).12
Genotyping of G308A gene polymorphismOligonucleotide primers and probes were designed with the Beacon Designer 4.0 (Premier Biosoft International®, LA, CA). The polymerase chain reaction (PCR) was carried out with 50 ng of genomic DNA, 0.5 uL of each oligonucleotide primer (primer forward: 5’-CTG TCT GGA AGT TAG AAG GAA AC3’; primer reverse: 5’-TGT GTG TAG GAC CCT GGA G-3’), and 0.25 uL of each probes (wild probe: 5’-Fam-AAC CCC GTC CTC ATG CCC-Tamra-3’) and (mutant probe: 5’-Hex-ACC CCG TCT TCA TGC CCC-Tamra-3’) in a 25 uL final volume (Ter-mociclador iCycler IQ (Bio-Rad®), Hercules, CA). DNA was denaturized at 95 °C for S min; this was followed by 50 cycles of denaturation at 95 °C for 15 s, and annealing at 59.S° for 45 s). The PCR were run in a 25 uL final volume containing 12.5 uL of IQTM Supermix (Bio-Rad®, Hercules, CA) with hot start Taq DNA polymerase.
Statistical analysisSample size was calculated to detect differences over 1.5 units of HOMA with 90% power and 5% significance (n = 60). The statistical analysis was performed for the combined GS08A and AS08A as a mutant group and wild type GS08G as second group. (Dominant model). The results were expressed as average ± standard deviation. The distribution of variables was analyzed with Kolmo-gorov-Smirnov test. Quantitative variables with normal distribution were analyzed with a two-tailed Student’s-t test. Non-parametric variables were analyzed with the Mann-Whitney U test. Qualitative variables were analyzed with the chi-square test, with Yates correction as necessary, and Fisher’s test. A logistic regression model (step by step) was used to study the histological variables as dependent endpoint and its relation with other independent variables associated in univariate analysis, all the models adjusted by age, sex, body mass index and genotype. Hardy-Weinberg equilibrium was assessed. A p-value under 0.05 was considered statistically significant. SPSS 15.0 software was used.
ResultsSixty six patients gave informed consent and were enrolled in the study. The mean age was 43.6 ± 12.2 years and the mean BMI 34.5 ± 6.5 with 50 males (75.5%) and 16 females (24.5%). Fifteen patients (4 females/11 males) (22.7%) had the genotype G308A (mutant type group) and 51 patients (12 females/39 males) (77.3%) G308G (wild type group). AA genotype was not detected.
In the control group, the mean age was 45.8 ± 6.4 years and mean BMI was S4.5 ± 4.6. There were 43 females (20.2%) and 160 males (79.8%). A total of 154 patients (30 females/124 males) (75.5%) had the genotype G-308G (wild type group) with an average age of 43.8 ± 16.7 years and 49 patients (13 females/ 36 males) (24.5%) had the genotype G-308A (mutant type group) with an average age of 45.3 ± 15.2 years, without statistical differences. The distribution of 308 genotype in NAFLD and control subjects was similar.
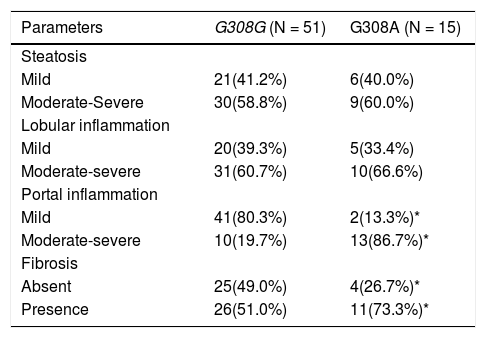
Tables 1, 2 and 3 shows the findings according TNF-alpha genotype. Table 1 shows the anthropo-metric variables in wild and mutant type groups. No differences were detected in fat mass or other an-thropometric parameters. Table 2 shows the differences in cardiovascular risk factors. Glucose levels, TNF-alpha and HOMA were higher in mutant type group than wild type group. No differences were detected in other parameters. Table 3 shows the histo-logical lesions in relation to both genotypes. Patients with mutant type group presented more moderate-severe portal inflammation (86.7%) in liver biopsy compared to patient with wild genotype (19.7%). Mutant type group had more fibrosis (73.3%) than wild type group (51.3%). In the next step of analysis, anthropometric and biochemical parameters are analyzed according liver histological classification. Patients with moderate-severe portal inflammation had higher levels of TNF alpha (7.4 ± 4.1 pg/mL vs. 4.9 ± 3.1 pg/mL vs: p < 0.05), HOMA (6.2 ± 3.1 vs. 3.8 ± 2.4 vs: p < 0.05), fat mass (30.5 ± 8.2 kg vs. 22.6 ± 7.9 kg: p < 0.05) and BMI (39.8 ± 11.1 kg/m2vs. 30.1 ± 6.1 kg/m2: p < 0.05) than patients with mild portal inflammation. After univariate analysis, we performed a multivariate analysis. The multivariate analysis adjusted by age, sex, BMI and genotype with the dependent variable (portal inflammation) showed that HOMA remained in the model, with an increase of the probability to develop moderate-severe inflammation of 1.45 (CI95%:1.02-2.1) with each increase of one unit on HOMA levels.
Changes in anthropometric variables.
| Parameters | G308G (N = 51) | G308A (N = 15) | P |
|---|---|---|---|
| BMI | 34.1 ± 9.4 | 35.5 ± 2.5 | 0.45 |
| Weight (kg) | 95.8 ± 27.7 | 92.1 ± 27.7 | 0.34 |
| Fat mass (kg) | 25.7 ± 8.9 | 23.6 ± 6.5 | 0.27 |
| Waist circumference (cm) | 101.6 ± 13 | 104.2 ± 3.5 | 0.56 |
| Waist to hip ratio | 0.95 ± 0.1 | 0.96 ± 0.1 | 0.71 |
(NS) p>0.05, between groups..
Classical cardiovascular risk factors.
| Parameters | G308G (N = 51) | G308A (N = 15) | p |
|---|---|---|---|
| Glucose (mg/dL) | 107.4 ± 25 | 131.6 ± 31.1* | 0.01 |
| Total ch. (mg/dL) | 196.1 ± 51.4 | 206.7 ± 51.3 | 0.65 |
| LDL-ch. (mg/dL) | 121.2 ± 45.3 | 132.3 ± 39.4 | 0.47 |
| HDL-ch. (mg/dL) | 50.1 ± 13.63 | 52.8 ± 20.7 | 0.24 |
| TG (mg/dl) | 145.6 ± 96 | 130.8 ± 59.8 | 0.29 |
| Insulin (mUI/L) | 14.8 ± 10.2 | 15.5 ± 9.2 | 0.11 |
| HOMA | 3.9 ± 2.7 | 5.9 ± 2.8* | 0.02 |
| TNF alpha (ng/mL) | 4.7 ± 3.2 | 7.4 ± 3.6* | 0.01 |
Ch.: Cholesterol. TG: Triglycerides. HOMA: Homeostasis model assessment. (*) p < 0.05, between groups.
Histological parameters.
| Parameters | G308G (N = 51) | G308A (N = 15) |
|---|---|---|
| Steatosis | ||
| Mild | 21(41.2%) | 6(40.0%) |
| Moderate-Severe | 30(58.8%) | 9(60.0%) |
| Lobular inflammation | ||
| Mild | 20(39.3%) | 5(33.4%) |
| Moderate-severe | 31(60.7%) | 10(66.6%) |
| Portal inflammation | ||
| Mild | 41(80.3%) | 2(13.3%)* |
| Moderate-severe | 10(19.7%) | 13(86.7%)* |
| Fibrosis | ||
| Absent | 25(49.0%) | 4(26.7%)* |
| Presence | 26(51.0%) | 11(73.3%)* |
Chi square test, differences in each stage(*)p<0.05. (%) frequencies in each genotype group, the amount of mild and moderate-severe damage is 100%.
Patients with presence fibrosis had higher levels of HOMA (4.1 ± 2.3 vs. 2.9 ± 1.7: p < 0.05), fat mass (23.4 ± 8.6 kg vs. 28.2 ± 8.4 kg: p < 0.05) and BMI (29.8 ± 5.2 kg/m2vs. 35.3 ± 10.8 kg/m2: p < 0.05) than patients without fibrosis. The multivariate analysis adjusted by age, sex, BMI and genotype with the dependent variable (fibrosis) showed that HOMA remained in the model, with an increase of the probability to develop fibrosis of 1.78 (CI95%:1.06-3.2) with each increase of one unit on HOMA levels.
The univariate analysis of anthropometric and biochemical parameters by steatosis and lobular inflammation does not show statistical differences.
DiscussionThe present study demonstrates that G308A polymorphism of TNF-alpha is associated with insulin resistance, TNF alpha, portal inflammation and liver fibrosis in patients with NAFLD. In logistic regression analysis, only insulin resistance was associated with portal inflammation and fibrosis.
The polymorphism at position-308(TNF-308 G-> A) leads to a higher rate of TNF alpha gene transcription, followed by raised TNF alpha concentrations and decreased insulin sensitivity.13 The mechanism of insulin resistance involves down regulation of PPARgamma, which have been shown to be the propagators of adipocyte differentiation.14 Other mechanism is the down-regulation of GLUT-4 or other cellular components that mediate the metabolic effects of insulin.15 Marchesini et al.15 demonstrated a closely correlation between insulin resistance (HOMA) and NAFLD, too. Other authors have been detected this relation using the clamp te-chnique16-18 with results supporting our conclusions. The nature of the connection between insulin resistance and hepatic steatosis remains unclear.19 In obese patients, the primary abnormality may be genetically induced insulin resistance, with a secondary increase of serum triglyceride levels due to enhance of peripheral lipolysis. The resulting hepatic supply of fatty acids and insulin may increase tri-glyceride deposition in the liver.20,21
TNF alpha has also a central role in the development of fatty liver and subsequently NASH. Elevated circulating TNF alpha levels area associated with obesity and insulin resistance in humans.22 It was suggested that TNF alpha profibrotic action is mediated through Kupffer cells activation.23 Treatment of leptin-deficient mice with TNF alpha antibodies improved hepatic insulin resistance and fatty liver.24 However, results are contradictory in the case of TNF alpha in humans; it might reflect different study populations or the lack of adjustment for several factors that may influence its relationship, for example genetic background of the study population. Hui et al.5 showed that although TNF alpha levels significantly differed between NALFD and controls, no such difference existed between NASH and NAFLD. In a study of 23 patients with NASH, 21 with NAFLD and 18 controls, serum TNF alpha and TNF receptor 1 were significantly increased in NASH compared to both controls and NAFLD.25 However, no significant difference of serum TNF alpha levels was found between a population of non-obese non diabetic NASH patients and matched controls.26 Perhaps, these different results could be explained by inclusion criteria and heterogeneity of subjects (age, basal BMI, presence of diabetes melli-tus, genetic background) in the various studies of the literature and our study.
Moreover certain genotypic effects could be population-specific. Furthermore, the 238 TNF alpha polymorphism has been found significantly more frequently in patients with fatty liver than healthy controls27 and the 1031 and 863A polymorphisms in NASH than fatty liver.28 In alcoholic Spanish men, 238GA polymorphism of TNF alpha was associated with alcoholic liver cirrhosis.29 Carulli et al.30 have demonstrated an association between interleukin-6-174G/C polymorphism and NAFLD. However, our study has limitations. First, the sample size of our design is small. Secondly, the cross sectional design of our study. This design is unable of explain causality.
In conclusion, G308A genotype is associated with high insulin resistance and TNF-alpha levels than G308G genotype. Patients with mutant genotype have more frequently moderate-severe portal inflammation and fibrosis than wild type genotype. Insulin resistance may be the biochemical nexus, because HOMA remained in an adjusted logistic regression model. We suggested that TNF alpha genetic poly-morphisms, involved in inflammation and insulin resistance, are associated with NAFLD, and this association could contribute to the understanding of the genetic susceptibility to NAFLD.