Introduction. Heparin having anti-inflammatory and anti-fibrotic properties may have therapeutic effect on liver injury. The present study investigated the effect of low molecular weight heparin (Enoxaparin) on carbon tetrachloride (CCl4) induced hepatic necrosis and apoptosis in rats.
Material and methods. Thirty male rats were divided into 5 groups. Group I: Control; Group II: Olive oil dissolved CCl4 at dose of 1 mL/kg, ip, twice per week; Group III: CCl4 and Enoxaparin at dose of 180 lU/kg, sc, daily; Group IV: Enoxaparin; Group V: Olive oil at dose of 1 mL, ip, twice per week. The liver histology at the forth week was examined by haematoxylin-eosin, Masson’s trichrome, Toluidine blue and Periodic acid schiff stains. Proliferative and apoptotic activities were assessed semi-quantitatively by proliferating cell nuclear antigen (PCNA) and cas-pase-3 immune staining and TUNEL method. Semi-quantitative values formulated by the equation HSCORE = ΣP( including both distribution and intensity of staining. Additionally, nidogen and α-smooth muscle actin were labeled by immunohistochemistry.
Results. CCl4 group had marked hepatocelluar necrosis around the vena centralis and increased inflammatory cells and mast cells. Hepatocytes showed deposition of lipid droplets, decrease in glycogen, apoptosis, and picnotic or enlarged nuclei. Enoxaparin reduced necrosis, apoptosis, and number of mast cells but had no effect on lipid droplets in hepatocytes. HSCORE’s of caspase-3 and PCNA were also significantly decreased by administration.
Conclusion. Enoxaparin have beneficial effects against necrosis as well as apoptosis at the early stage of CCL4 induced liver injury.
The capacity of regeneration distinguishes the liver from the other essential organs in adults. Incomplete regeneration known as hepatic fibrosis is now regarded as a common response to liver injury and, regardless of its origin (viral infections, alcohol abuse, metal overload), is characterized by excessive accumulation of extracellular matrix (ECM) compo-nents.1–4 Emerging experimental evidence indicates that oxidative stress could represent a common link between the different types of chronic liver injuries and hepatic fibrosis. In fact, lipid peroxidation has been associated with liver fibrosis due to iron overload, ethanol, carbon tetrachloride, and virus C he-patitis.4–8 Independent stimulation of ECM deposition seems to occur at a prenecrotic stage during oxidative stress-associated liver injury, although it is difficult to discriminate between profibrogenic and true fibrogenic effects induced by free radical species. Hepatic stellate cells (HSCs) are a major source of ECM in normal and pathological conditions. During fibrogenesis, HSCs undergo a process of activation, developing a myofibroblast-like phenotype associated with increased proliferation and collagen synthesis.9 Activation of stellate cells is regulated by several soluble factors, including cytokines, chemokines, growth factors (hepato-cyte growth factor, epidermal growth factor, transforming growth factor), and also products of oxidative stress.4,10,11
Mast cells are distributed in all organs including liver which have pro-fibrogenic and anti-fibrotic mediators. They are multi-effector cells with important roles in the tissue destruction and remodeling in liver injury.12–15
CCl4 is known to be hepatotoxic as well as ne-phrotoxic to humans.16,17 CCl4 induces necrosis and apoptosis of the liver in a variety of experimental animal models.5,9 While a single oral, intraperito-neal, subcutaneous dose of CCl4 will lead to reversible liver injury, a prolonged exposure will result with fibrosis, cirrhosis and hepatic carcinoma.2,18,19
Treatment of liver fibrosis still remains as a therapeutic challenge. There is a need for a therapy that will decrease the extent of fibrosis or enhance the liver regeneration. Few treatments have been proposed to alter the hepatocyte regenerative activity but none has proven clinically effective.6,13,19–21
Low-molecular-weight heparins (LMWH) are fragments of commercial grade heparin produced by either chemical or enzymatic depolymeriza-tion. Deaminative cleavage with nitrous acid is used in the manufacture of dalteparin, reviparin and nadroparin. Alkaline beta-eliminative cleavage of the benzyl ester of heparin is used in the manufacturing of Enoxaparin. LMWHs are potentially more advantageous pharmacologically and pharmacokinetically than heparin due to their reduced hemorrhagic to antithrombotic ratio, lesser risk of bleeding, greater bioavailability at low doses, and longer half-life.22 The antifibrotic role of a LMWH was shown as an experimental model of glomerulotoxicity and lung fibrosis.17,22,23 In en-dotoxin-induced liver injury, Dalteparin reduces sinusoidal capillarization and the production of inflammatory cytokine in Kupffer cells.24 Additionally, recent clinical data have suggested a beneficial effect for LMWH as hepatic antifibrotic agent.8,25–28
The present study aimed to examine antifibrotic and regenerative effects of LMWH on CCl4 induced toxic liver injury by immunohistochemistry with light and electron microscopy.
Material And MethodsAnimalsThe present study was approved by the laboratory animals ethic comitee of university (2005/30039). Experiments were carried out on 30 Sprague-Dawley male rats of 260-320 g body weight. They were housed at 22-24 °C and were exposed to alternate cycles of 12 h light and darkness. They were given free access to a standard pellet diet and tapwater.
Experimental procedureThe animals were divided into 5 groups with 6 rats in each group. Group I was control rats. Group II was administered CCl4 (Merck) at a dose of 1 mL/kg of body weight, intraperitoneal, dissolved in olive oil, twice per week for four weeks. Group III had CCl4 and Enoxaparin (Enoxaparin Sodium, Aventis Pharma) 180 IU/kg of body weight, subcutaneously daily. Group IV was given Enoxaparin 180 IU/kg of body weight subcuta-neously daily. Group V was administered olive oil at a dose of 1 mL, ip, twice per week. At the end of four weeks, animals were anesthetized with keta-mine/xylacine (90/10 mg/kg) and sacrified by decapitation. After removal and weighing the livers were placed in 10% formalin and 2.5% glutaralde-hyde for histological processing.
Light microscopyLiver samples were fixed in 10% formalin, routinely processed and embedded in paraffin wax. Sections of 5 micrometer thickness were cut and stained with haematoxylin and eosin (HE), Masson’s trichrome for collagen, Toluidine blue for mast cells, Periodic acid schiff (PAS) for glyco-gen and by immunohistochemistry to demonstrate caspase-3, proliferating cell nuclear antigen (PCNA), nidogen (entactin) and smooth muscle α-actin (α-SMA) for evaluation of parenchymal and non-parenchymal cells. Photographs were taken using an Olympus BX 50 photomicroscope. Mast cells were counted in ten sections by using an eyepiece micrometer (OC-M, Olympus, Japan, X40) all portal areas in the section.
Electron microscopyLiver tissues were fixed in 2.5% glutaraldehyde in 0.1 M sodium phosphate buffer, tissues were pos-tfixed with 2% osmium tetraoxide in sodium phosphate buffer. Dehydration was accomplished by gradual ethanol series and tissues were embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate. Sections were then viewed and photographed with a Zeiss 9EM.
Caspase-3, PCNA, nidogen, and α-SMAThe liver tissue samples were fixed in 10% neutral formaline for 24 h, embedded, and then serial sections (5 μm) were collected on slides with polysi-ne. After rehydrating, samples were transferred to 0.01 M citrate buffer (pH 6) and subsequently heated twice in a microwave oven for 5 min each time at 750 W. for antigen retrieval. After cooling for 20 min at room temperature, the sections were washed with phosphate buffer saline (PBS). To remove endogenous peroxidase activity, sections were kept in 3% H2O2 for 20 min and afterward washed with PBS. Sections were incubated with primary rabbit-po-lyclonal Caspase-3 antibody (NeoMarkers, Fremont, CA) and primary mouse-monoclonal PCNA antibody (Dako, Carpinteria, CA), primary mouse anti-smooth muscle Actin (anti-α-SMA Zymed Lab., Lab Vision, USD). Some sections were incubated with primary goat-polyclonal nidogen-1 and nidogen-2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 1:100 dilution overnight at 4 °C. Negative control sections were treated with nonimmune serum diluted in the same manner. Labeling was visualized using the Universal LSAB kit (Dako, Carpinteria, CA) according to the manufacturer’s instructions. Staining was completed with DAB Chromogen (Dako, Carpinteria, CA) for 1-2 min, and slides were counterstained with Harris’s Haematoxylin, dehydrated and then cover-slipped with permount.
Detection of apoptotic cellsApoptotic hepatocytes were detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end-labeling (TUNEL) staining using a commercial ready-to-use kit (Cat No MK500, Takara Bio Inc. Japan, In situ apoptosis detection kit) according to the manufacturer’s recommendations. Randomly selected different areas of six sections per group at X 400 magnification were evaluated for the analysis of TUNEL staining. The apoptotic index (AI) was determined as the percentage of labeled cells (TUNEL positive) with respect to the total number of cells counted using the formula: AI = (Number of labeled cells/Total number of cells counted) x 100.19
Semiquantitative evaluationSections were evaluated with respect to Cas-pase-3, and PCNA localization in a semiquantitative manner using a light microscope and se-lected areas were photographed. Two experienced observers unaware of the treatment were scored hepatocyte staining intensity as negative (-), weak (+), moderate (+ +), strong (+ + +). HSCORE values of Caspase-3 and PCNA staining were obtained in a semiquantitative manner and included both intensity and distribution patterns of staining.29 Ten different areas of six sections per group at X 400 magnification were evaluated for the analysis of immunohistochemi-cal Caspase-3 and PCNA staining. Values were recorded as percentages of positively stained target cells in each of four intensity categories which were denoted as 0 (no staining), 1 + (weak), 2+ (moderate), 3+ (strong). For each tissue, an HSCORE value was derived by summing the percentages of cells that stained at each intensity category and multiplying that value by the weighted intensity of the staining, using the formula HSCORE = ΣPi (i + l), where i represents the intensity scores and Pi is the corresponding percentage of the cells.
Statistical analysisComparisons of the HSCORE values and AI were performed by the One-Way ANOVA-Tukey’s test. Number of mast cells were analysed by Mann-Whit-ney-U test. Data were expressed as mean ± SD. P values less than 0.05 were considered to be statistically significant.
ResultsLight microscopy, electron microscopy and immunohistochemistryHistologic findings of the groups to which only Enoxaparin (Group IV) and olive oil (Group V) were administered were close to the controls. In the CCl4 administered group, wide areas of necrosis around the central vein, hepatocytes with picnotic and large nuclei and lipid accumulation were noted (Figures 1A and C). Engorgement of the sinusoids within the necrotic fields, hemorrhage and increase in inflammatory cells were observed (Figure 1B). Clusters of Kupffer cells (Figure 1D), apoptotic he-patocytes and mitotic hepatocytes (Figure 1C) were identified. In the Enoxaparin-treated group, enlargement of the sinusoids in some areas and persistence of lipid droplets in hepatocytes were observed though necrotic areas did not appear (Figures 1E and F). Mast cells were seen around the portal area. CCl4 exposure for four weeks also caused significant increase in the number of mast cells in portal area (Figure 1G) which was decreased by Enoxaparin (P <0.01, Figures 1H and 2).
Masson’s trichrome, HE and Toluidine blue stainnings. CCl4 group, wide spreading necrosis, Masson’s trichrome x10 (A), increase in inflammatory cells (→), HE x10 (B), mitotic figure (→), HE x40, (C), activation of Kupffer cell (→), Masson’s trichrome x40 (D). Increase in mast cells, Toluidine blue stainning X 40 (G). Enoxaparin prevented CCL4 induced necrosis, HE x20 (E). Enoxaparin treatment caused vacuolization in the hepatocytes result of melting lipid droplets HE x20 (F). Enoxaparin prevented CCL4 induced mast cell increase, Toluidine blue stainning X 40 (H).
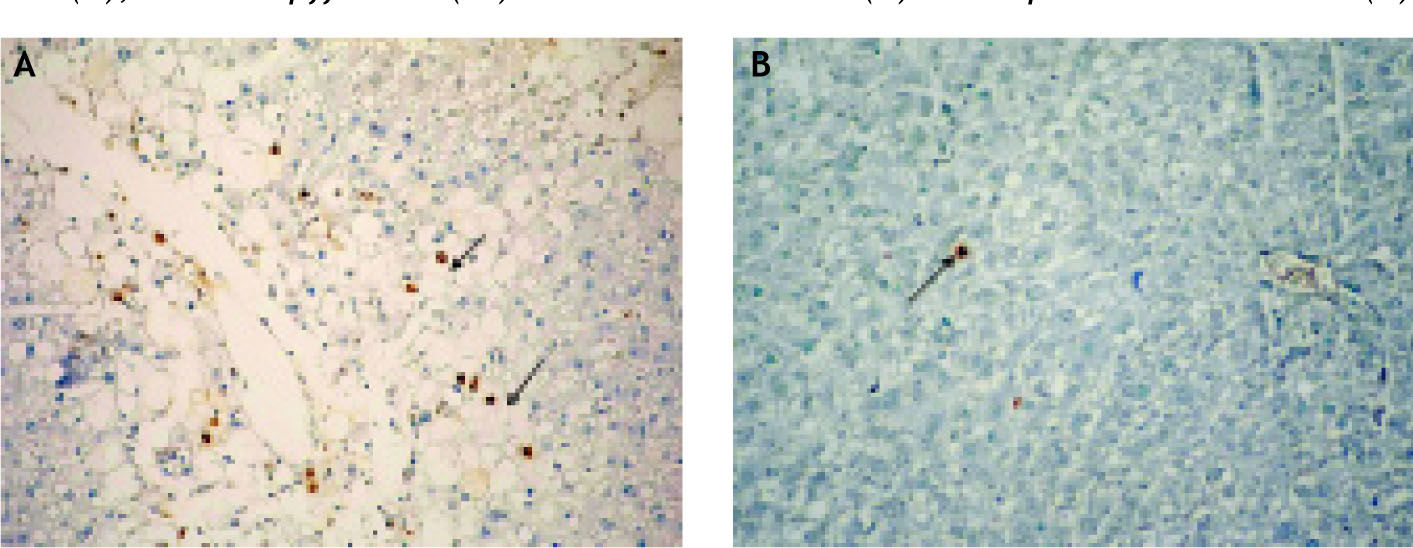
When the glycogen content of the hepatocytes was examined with respect to the controls, we found that staining of the cells around the necrotic areas was weaker and glycogen content was reduced (Figure 3A) in the CCl4-administered group whereas there were numerous PAS-stained hepato-cytes in the Enoxaparin-administered group (Group III) and the staining intensity was comparable to the controls (Figure 3B). By the portal areas and around the central veins in the CCl4-ad-ministered group, fibrosis was not detected. When the semi-thin sections were examined, necrosis, va-cuolization in the hepatocytes, lipid accumulation and Kupffer cells were clearly identified (Figures 4A, B and C). TUNEL staining of the tissues demonstrated significant increase in the CCl4 and sig-nificant decrease in the Enoxaparin-administered groups in the apoptotic hepatocytes compared to the controls (Figures 5 and 6).
α-SMA (Figures 7A, B and C) was comparable in the control and other groups. α-SMA was present around the vessels in the portal areas and did not stain the HSCs. Immunohistochemical labeling of nidogen of groups were decreased in the bile duct and endothelial during acute hepatic CCL4 injury (Figures 7D, E and F). When the fine structure was examined, mitochondria and rough endoplasmic re-ticula of the hepatocytes appeared normal in the control group (Figure 8A). In the CCl4– administered group, on the other hand, the boundaries of the cells were indistinguishable, the organelles were disrupted and there were necrotic changes (Figure 8B) and apoptotic hepatocytes (Figure 8C). In the Enoxaparin-administered group, necrotic areas were less, but lipid deposits in the hepato-cytes persisted (Figure 8D). In the necrotic areas, active hepatic stellate cells which transformed into myofibroblasts were not identified. Presence of increased collagen fibers was not observed around the sinusoids in the portal areas.
Semi-quantitative HSCORE values gathered during the assessments of the groups in terms of Cas-pase-3, PCNA, and nidogen are shown in table I, data are expressed as mean±standart derivation. The CCl4-administered group, in comparison to the all other experimental groups, depicted significant increase in Caspase-3 (28.88 ± 3.42), and PCNA (205.60 ± 24.27) values in hepatocytes (P < 0.05). Increases in PCNA, which is a marker of proliferation, and Caspase-3, which plays a key role in apop-tosis, suggest that CCL4 increases mitotic activity and apoptosis in hepatocytes at the same time. HS-CORE values were significantly lower in the group treated with Enoxaparin than the CCl4 –administered group (P < 0.05) but significantly higher than the group that received only Enoxaparin and the controls. Enoxaparin also prevented decreasing ni-dogen expression in bile ducts (Table 1.
HSCORE of Caspase-3 and PCNA immunostainings (Mean ± SD).
| Groups (n = 6) | Caspase-3 (Hepatocyte) | PCNA (Hepatocyte) | Nidogen (Bile duct) |
|---|---|---|---|
| Control | 3.60 ± 1.67 | 7.60 ± 3.29 | 400 ± 0.00 |
| Olive oil | 2 ± 1.41 | 8.40 ± 2.61 | 400 ± 0.00 |
| CCl4 | 28.88 ± 3.42* | 205.60 ± 24.27* | 300 ± 0.00* |
| CCl4 + Enoxaparin | 17.6 ± 3.91* | 153.60 ± 30.8* | 400 ± 0.00 |
| Enoxaparin | 10.88 ± 1.19 | 14.8 ± 3.63 | 400 ± 0.00 |
* Significantly different from all other groups (P <0,05).
There was no significant difference in body and hepatic weight between the experimental groups and controls.
DiscussionThe liver injury model with CCl4 was used to study effect of Enoxaparin at early stage of chronic liver injury. Enoxaparin was beneficial on hepatocytes both for preventing apoptosis and improving regeneration which may be the mechanisms of Enoxaparin for preventing liver fibrosis. However, rats did not developed liver fibrosis with this experimental model, and so chronic treatment of Enoxaparin should be studied to settle role of Enoxaparin on preventing liver fibrosis.
In the normal adult liver, hepatocytes are in a quiescent state and turn over very slowly [1–2 times/year]. However, the liver regenerates very quickly during acute toxic liver injury in rodents, a similar process occurs at a somewhat slower rate in larger animals and in humans.30 There are two stages of hepatic injury, namely regeneration stage and fibrosis stage. In the regeneration stage, cells of the original type appear in the injured tissue. In the fi-brosis stage, normal parenchyma is replaced by connective tissue and ECM accumulation resulting with scar formation. HSCs play important and deterministic roles at this stage. The recent studies have focused on preventing fibrosis by reducing the activated HSCs.1,31
CCI4 has long served as a model for study of he-patotoxicity with centrilobular hepatic necrosis. It is accumulated in hepatic parenchyma cells and metabolized to CCl3 by liver cytochrome P450-depen-dent monooxygenases. Metabolic activation of CCl4 to free radicals, namely trichloromethyl and tri-chloromethyl peroxy radicals, is reported to enhance lipid peroxidation and protein oxidation leading to liver injury.16,19 Acute injury as a result of single dose of oral, intraperitoneal or subcutaneous CCl4 administration is usually reversible and full regeneration can be achieved.2,8 Fibrosis and pseu-dolobulation occur when the duration of administration is prolonged. The number of α-SMA (+) cells increases in the fibrotic areas. These changes are reported to recover with Vitamin-A administration.20
In the present study, CCl4 was administrated to rats every other day for 4 weeks caused necrosis, li-pid peroxidation reduction in glycogen in the hepa-tocytes, inflammation and activation of Kupffer cells but not fibrosis. As a result, we did not observe HSCs stained by α-SMA. The number of mast cells in portal area also increased in early stage of liver injury by induced CCL4 which is parallel with other studies.13–15 Mast cells have a significant role in tissue destruction and remodelling in liver injuries by releasing vasoactive, nociceptive and pro-inflammatory mediators. Matsunaga, et al., found that in livers with chronic diseases, the densities of tryptase+ mast cells, chymase+ mast cells, and S-100+ nerve fibers in the stroma were significantly higher than of the ones in normal livers. They observed a significant increase of tryptase+ and chy-mase+ mast cells and also innervation in correlation with degree of fibrosis.14
CCl4 induces massive necrosis in the liver and consequent oxidative stress activates caspase-3 and increases apoptosis. Increase in apoptotic hepato-cytes has been described in hepatic fibrosis and viral hepatitis.9,21,27,32 Although apoptosis of HSCs shown to have a peak at 64h,9 in another study apoptosis started to increase at 2 weeks and made a peak at 4 weeks.21 The recent study evaluated only the apop-tosis of hepatocytes which increased in a dose-dependent fashion. Apoptosis scores were found to be 16.76 ± 1.1 and 25.55 ± 1.96 after the single CCl4 doses of 20 and 50 μl/kg, respectively.19
In addition to apoptosis, increase in PCNA-labe-ling was also noted in the hepatocytes of CCl4–admi-nistered rats.33 PCNA score was reported as 6.4 ± 1 in a control group while that was 19.7 ± 2.0 a day after CCl4 administration.34 TUNEL method was used to demonstrate increase in apoptotic hepato-cytes and Caspase-3 immune-staining was used to identify hepatocytes that will undergo apoptosis. PCNA score as an indicator of proliferation was increased significantly in the groups with necrosis as opposed to the control group. There was an 8-fold increase in apoptosis and 27-fold increase in proliferation in comparison to the control group. In acute injury, the response of the hepatocytes is significantly more prominent and cells prepare for mitotic division. On one hand, there is elimination of hepatocytes affected from CCl4 by apoptosis and, on the other hand, there is an increase in the prolifera-tion of un-affected hepatocytes.
In the normal adult liver, basal lamina is present in the bile ducts, arteries and veins, but not in sinusoids. Laminin, nidogen (entactin), and Type IV collagen increase/accumulate and a basement membrane-like structure forms in the space of Disse during hepatic regeneration and fibrosis, although an organized basal lamina does not develop.35,36
Immunohistochemical labeling of nidogen of groups were decreased in the bile duct and endothe-lial during acute hepatic CCL4 injury. Since HSCs were not activated after 4 weeks of CCl4 administration, nidogen expression and collagen were not increased. Presence of α-SMA staining only around the vessels and absence of HSCs lend also support to this result. Thus, this liver injury model used in the present study was an early stage of chronic damage.
Numerous drugs and herbal substances have been tried in the treatment of hepatic injury and fi-brosis. To date, there is no routinely used treatment modality. Both in-vitro and in-vivo experimental studies showed heparin has anti-fibrotic activity in liver and also other organs.8,17,22–26 Heparin has been shown to inhibit growth of HSCs which play important roles in fibrosis.37 Signal transduction pathway of Heparin is related to activities of extracellular signal regulated kinase (ERK) and down regulation of activator protein 1 (AP-1).26
The effects of LMWHs were investigated in cho-lestatic liver injury model and Nadroparin and Enoxaparin have been shown to decrease serum bi-llirubine level, alleviate degeneration and reduce fi-brosis.27 Enoxaparin has been shown to have both anti-inflammatory and antifibrotic activities. Enoxaparin reduced hepatic fibrosis in thioacetami-de-induced cirrhotic rats.25 Harada, et al., showed that, dalteparin, a LMWH, protects liver from ische-mia/reperfusion-induced injury by inhibiting leukocyte activation. They suggested that therapeutic effect of Dalteparin might be independent of its anticoagulant activity but dependent on its capacity to enhance endothelial production of prostacyclin via cyclooxygenase-1 activation.38
Recently, Dalteparin sodium was used in CCl4-in-duced fibrosis for 7 weeks and dose-dependent increases in PCNA score of hepatocytes and in apop-tosis of cultured HSCs were observed.8 They found that, necrosis around the central vein and inflam-matory cell infiltration in the middle zone occurs 24 h after CCl4 administration which was not prevented by Dalteparin. There was an increase in PCNA (+) labelled hepatocytes and the increase in non-pa-renchymal cells appeared 72 h later. Dalteparine diminished α-SMA staining and fibrotic bands formation which occured 7 weeks after CCl4 administration. It has been reported that dalteparine inhibits the profibrogenic response due to chronic CCl4 administration, stimulates Hepatocyte Growth Factor (HGF), down-regulates Transforming growth factor (TGF)-beta and directly inhibits the proliferation of the HSCs.8 Heparin functions as a hepatotrophic factor by inducing production of HGF.10,11In vitro studies showed that addition of heparin to cultures of human fibroblasts, leukemic cells, and umbilical vein endothelial cells stimulates HGF production to 3-6-fold higher levels than seen in the absence of heparin. Administration of heparin to rats increased blood HGF to 2.5-5 fold higher levels than controls given saline alone. Consequently, a remarkable enhancement of in-vivo liver regeneration was induced after 30% partial hepatectomy.25 Additionally, Shi, et al., showed anti-fibrotic effect of LMWH in clinical trial involving 34 patients with chronic hepatitis.28
In the present study, necrosis around the central vein of the hepatic tissue was found markedly improved in rats treated with Enoxaparin, which is a LMWH. Enoxaparin prevented increase of mast cells which was induced by CCL4. Decrease in the number of activated Kupffer cells and increase in the number of PAS positive-stained hepatocytes were also observed. By preventing activation of Kupffer cells, release of pro-inflammatory agents decrease and tissue damage can be hindered. This beneficial effect of Enoxaparin was also evident on apoptosis and HS-CORE of PCNA. Enoxaparin did not totally prevent proliferation of hepatoctyes. Even though increased apoptosis and PCNA improved with Enoxaparin, it was still higher than the control group.
ConclusionEnoxaparin, a low molecular weight heparin, prevented inflammation in acute phase and possibly reduced the activation of Kupffer cells and mast cells, and demonstrated a protective role against necrosis and apoptosis. LMWH is cheap and safe, can be an option for treatment of liver injury if the further studies also confirm its beneficial effects on chronic liver injury.
Abbrevations- •
CCl4: Carbon tetrachloride.
- •
PCNA: Proliferating cell nuclear antigen.
- •
ECM: Extracellular matrix.
- •
HSC: Hepatic stellate cell.
- •
LMWH: Low-molecular-weight heparin.
- •
HE: Haematoxylin and eosin.
- •
PAS: Periodic acid schiff.
- •
α-SMA: Smooth muscle α-actin.
- •
PBS: Phosphate buffer saline.
- •
TUNEL: Terminal deoxynucleotidyl transfera-se-mediated dUTP nick end-labeling.
- •
AI: Apoptotic index.
- •
AP-1: Activator protein 1.
- •
ERK: Extracellular signal regulated kinase.
- •
HGF: Hepatocyte growth factor.
- •
TGF: Transforming growth factor.
This study is funded by Abant Izzet Baysal University Scientific Research Fund (2006.08.01.247) and was presented as a poster in the International Liver Congress 2010 by EASL, Vienna-Austria, April 14-18, 2010.



















![Immunostaining of Nidojen (A, B and C) ve α-SMA [D x 20, E x 40, F x 40]. Control group (A and D). CCl4 group (B and E). Enoxaparin treatment (C and F). Immunostaining of Nidojen (A, B and C) ve α-SMA [D x 20, E x 40, F x 40]. Control group (A and D). CCl4 group (B and E). Enoxaparin treatment (C and F).](https://static.elsevier.es/multimedia/16652681/0000000900000004/v1_201906230846/S1665268119316217/v1_201906230846/en/main.assets/thumbnail/gr7.jpeg?xkr=ue/ImdikoIMrsJoerZ+w96p5LBcBpyJTqfwgorxm+Ow=)





