Aminotransferase elevation is a frequent cause of consultation for the Hepatologist, in both the outpatient and inpatient settings, but identifying the origin of these biochemical alterations may be challenging. Here we report a case where acute elevation of aminotransferases, associated with abdominal symptoms, was the cause of two hospitalizations in a short period of time. As the patient suffered from type 1 diabetes, celiac disease, and autoimmune thyroiditis, several potential causes of damage could be hypothesized, including celiac hepatitis, fatty liver, and autoimmune hepatitis. A liver biopsy performed in the occasion of the second hospitalization allowed to rule out autoimmune hepatitis and celiac hepatitis, showing mild signs of fatty infiltration. Staining with periodic acid-Schiff with or without diastase showed a marked accumulation of glycogen, indicating the presence of a glycogenic hepatopathy associated with poorly controlled type 1 diabetes. This condition may be a cause of liver damage in patients with type 1 and occasionally type 2 diabetes, but its occurrence is often overlooked. This case report illustrates the fact that glycogenic hepatopathy may relapse, and prompts the clinician to take into account this condition in the differential diagnosis of causes of liver injury.
Aminotransferase elevation is a frequent cause of patient referral to the Hepatologist.1 In cases when there are associated symptoms, such as jaundice, fatigue, or upper gastrointestinal tract discomfort, the patient may even be referred to the emergency room and hospitalized. While the most common causes of liver injury, e.g. acute viral hepatitis, are well known and relatively easy to diagnose, in many cases the origin of the biochemical alterations may be difficult to ascertain. Identification of the etiology of hepatic abnormalities is critical to prevent progressive damage whenever the alterations persist, and to avoid recurrences in those that undergo normalization. In this paper, we report on a case of recurrent significant liver damage in a young woman, the origin of which could be potentially ascribed to different causes, and that was ultimately attributed to a glycogenic hepatopathy related to poorly controlled type 1 diitus.
Case ReportA 31 years old Caucasian woman was admitted to the emergency room because of worsening fatigue, epigastric pain associated with early satiety and dyspepsia, and abnormal liver enzymes at an outpatient control. She was transferred to the Medical Department for further testing. Her family history was unremarkable, in particular no history of cardiovascular or liver diseases was recalled. She had no siblings. At 6yrs of age she was diagnosed with type 1 diabetes mellitus and at the time of admission was being treated with three injections/day of insulin glulysine (10UI, 12UI, 10UI at each meal, respectively) and one injection/day of insulin glargine (12 UI). The patient was also followed up for diabetic retinopathy and neuropathy. Hashimoto’s thyroiditis was diagnosed when she was 16, and was treated with levothyroxine. When the patient was 20, a diagnosis of celiac disease was made based on the presence of positive anti-tranglutaminase antibodies and a compatible jejunal biopsy. She was prescribed a gluten-free diet. She recalled two hospital admissions in the past three years, one due to diabetic chetoacidosis and one to treatment-induced hypoglycemia. She had not taken any other prescription drugs in the last 6 months, and was not taking any OTC medications, or herbal compounds. She denied any alcohol consumption, and her parents, with whom she was living, confirmed this. At admission, physical examination revealed ankle edema, diffusely tender abdomen and hepatomegaly. Her body mass index was 25.2 and the waist circumference 96 cm. The remaining physical examination was unremarkable.
Laboratory testing confirmed elevated levels of aminotransferases (approximately 6x ULN) and gamma-glutamyltranspeptidase (GGT) (more than 20x ULN) (Table 1). Other relevant findings were an altered lipid profile and elevated glycated hemoglobin, compatible with a suboptimal control of diabetes. Upper abdominal ultrasound demonstrated a mild hepatomegaly and homogeneous parenchymal echotexture, possibly consistent with fatty infiltration.
Biochemical, immunological and virological testing at first admission.
| AST | 225 U/L (ULN 40) |
|---|---|
| ALT | 258 U/L (ULN 40) |
| Gamma-GT | 835 U/L (ULN 40) |
| ALP | 375 U/L (ULN 130) |
| Total bilirubin | 0.54 mg/dL (ULN 1.00) |
| Total cholesterol | 257 mg/dL (ULN 220) |
| LDL-cholesterol | Unreliable for triglycerides > 350 mg/dL |
| HDL-cholesterol | 52 mg/dL (NL > 35) |
| Triglycerides | 383 mg/dL (ULN 170) |
| TSH | 0.92 mU/L (NL 0.25 - 3.50) |
| Gamma-globulin | 14.5% |
| IgG | 10.70 g/L |
| Transferrin saturation | 28% |
| Ferritin | 195 ng/mL (NL 10-200 ng/mL), |
| Alpha1-antitrypsin | 1.02 g/L (NL 0.90-2.00 g/L) |
| Ceruloplasmin | 0.40 g/L (NL 0.20-6.00 g/L) |
| Urinary copper | 55 μg/24h (NL < 70 μg/24h) |
| Anti-tissue transglutaminase antibodies | Positive (high titer) |
| Virological markers | HCV-RNA negative |
| HBV markers and HBV-DNA negative | |
| HAV, HEV, EBV, CMV IgM negative | |
| Non-organ specific antibodies | ANA 1:160; AMA, ENA, ASMA, LKM1, ANCA negative |
| Pregnancy test | Negative |
| Hb A1c | 10.3% (ULN 5.5%) |
Because previous testing had repeatedly shown normality of aminotransferase and/or GGT, an extensive work-up was conducted to establish the cause of acute enzyme elevation (Table 1). Such tests allowed to rule out infection with major and minor hepatotropic viruses, primary biliary cirrhosis, and genetic liver diseases. Anti-nuclear antibodies were positive at low titer (1:160), but no hypergammaglo-bulinemia was present and the autoimmune hepatitis score was 9 (not probable).
The patient was discharged with a diagnosis of nonalcoholic fatty liver disease, mostly based on the ultrasound picture and dyslipidemia. Since the compliance of the patient to the gluten-free diet was suboptimal, the possibility of a celiac disease-related liver injury was also considered as an additional factor. The patient was recommended to improve her glycemic control and her adherence to a gluten-free diet. At follow-up after discharge, a marked reduction in the levels of aminotransferase and GGT was observed (Table 2).
Biochemical data at follow-up after the first hospital admission.
| 10 days later | 1 month later | 2 months later | |
|---|---|---|---|
| AST (ULN 40) | 60 U/L | 58 U/L | 46 U/L |
| ALT (ULN 40) | 92 U/L | 76 U/L | 54 U/L |
| Gamma-GT (ULN 40) | 228 U/L | 135 U/L | 97 U/L |
| ALP (ULN 130) | 134 U/L | 120 U/L | 115 U/L |
| Total bilirubin (ULN 1.00) | 0.51 mg/dL | 0.32 mg/dL | 0.29 mg/dL |
Five months later, she was again admitted to the hospital because of fatigue and liver enzymes indicating relapsing hepatic injury (AST 359 U/L, ALT 326 U/L, GGT 440 U/L and ALP 280 U/L). Total cholesterol was 263 mg/dL and triglycerides 359 mg/dL.
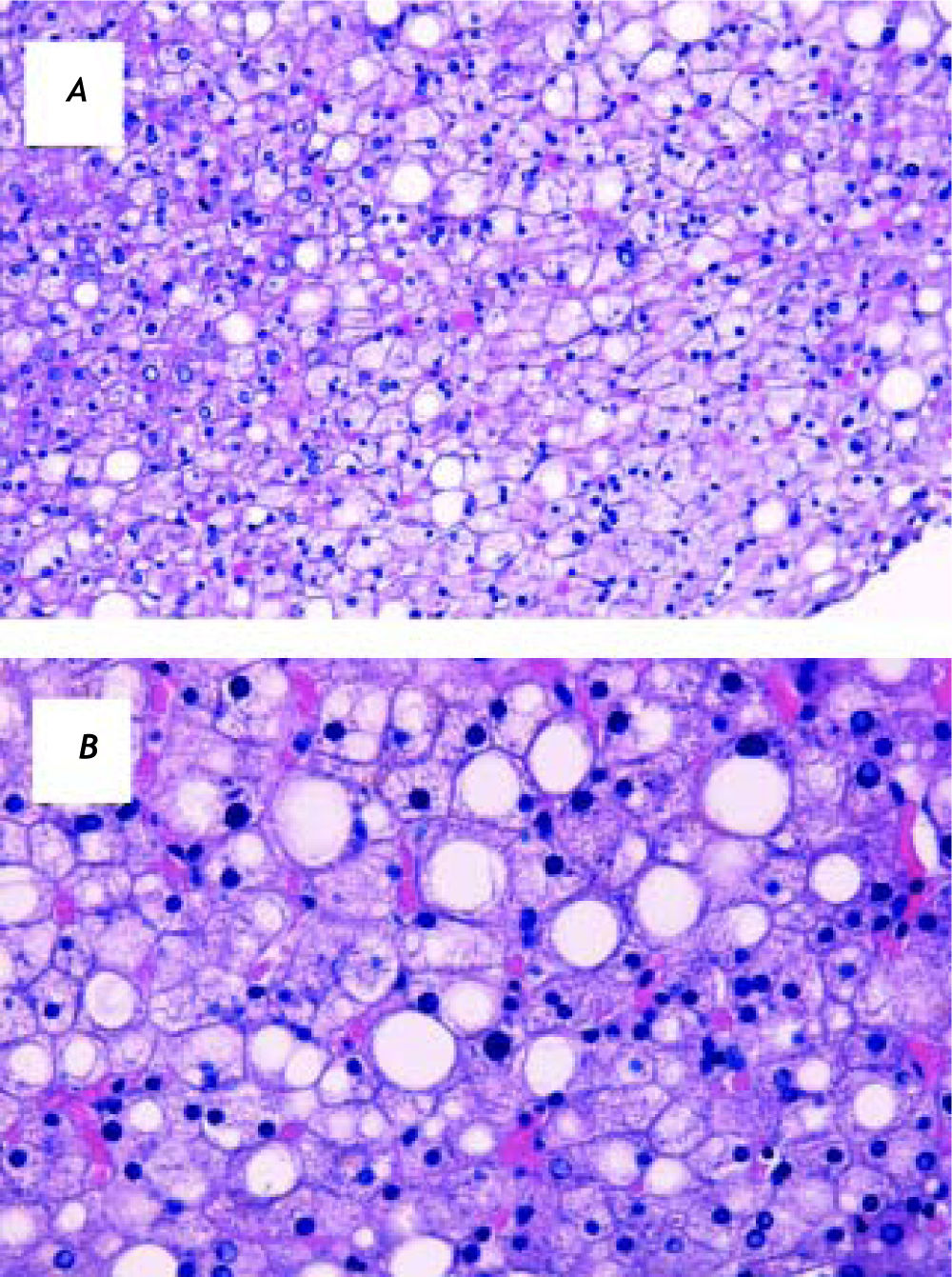
Viral infection markers were confirmed to be negative and tests of iron and copper overload were in the normal range. Autoantibodies (except for ANA 1:160) were negative. Abdominal ultrasound confirmed hepatomegaly and bright liver, suggestive for fatty infiltration. A liver biopsy was performed and showed macro-and microvescicular steatosis, involving 25% of hepatocytes, without significant inflammation, fibrosis or cholestasis (Figure 1). Perls staining was negative. Mallory hyaline bodies were not detected.
Liver biopsy, hematoxylin and eosin staining. Most of the hepatocytes appear to be enlarged and swollen, with pale cytoplasm and with polygonal shape. In addition, approximately 25% of hepatocytes show fat accumulation. No significant inflammation, fibrosis or cholestasis is present. Mallory hyaline bodies were not detected. A. Original magnification 20X. B. Original magnification 40X.
To obtain additional information on the potential cause of injury, the specimen was stained with Periodic Acid-Schiff (PAS) before and after diastase treatment (Figure 2). A diffuse cytoplasmic staining with PAS was observed, particularly intense in correspondence to the enlarged and swollen hepatocytes observed at hematoxylin and eosin staining. Notably, staining was no longer evident after diastase treatment, compatible with a marked accumulation of glycogen. PAS positive material was also found diffusely in the nucleus and as small densely stained round bodies (nuclear glycogen bodies) about the size of the nucleolus. The picture is typical of a glycogenic hepatopathy, which is associated with poorly controlled diabetes mellitus. After three months, the patient had achieved significant improvement in glycemic control, with normalization of liver enzymes.
Liver biopsy, Periodic acid-Schiff staining. A. PAS demonstrates a diffuse cytoplasmic staining, particularly intense in correspondence to the enlarged and swollen hepatocytes observed at hematoxylin and eosin staining. PAS positive material was also found diffusely in the nucleus and as small densely stained round bodies (nuclear glycogen bodies) about the size of the nucleolus. B. Staining was no longer evident after diastase treatment, compatible with a marked accumulation of glycogen. Original magnification 20X.
Glycogenic hepatopathy is defined as a pathologic overloading of hepatocytes with glycogen along with hepatomegaly and/or elevated liver enzymes in patients with long-standing poorly controlled diabetes mellitus.2 It is an underrecognized condition that may not easily be distinguished from nonalcoholic fatty liver disease (NAFLD) by history, physical examination or ultrasound, and only liver biopsy can ultimately provide the diagnosis.2 Unlike genetic glycogenosis, glycogenic hepatopathy is associated with a low risk of developing advanced liver disease and is easily treatable by improvement of glycemic control,3 and persistent reversal has also been observed following pancreas transplantation.4 Clinical presentation can include abdominal pain, sometimes associated with nausea and vomiting, hepatomegaly and abnormal liver function tests.2,3,5 The histological picture is characterized by a pale appearance of the hepatocytes with compression of the sinusoids, glycogenated nuclei, and giant mitochondria.2 Steatosis may be present, usually mild, or absent. Glycogen accumulation, the hallmark of this condition, is demonstrated by PAS-diastase staining.2,6
Glycogen accumulation in the liver has been described for the first time in 1930 as a component of Mauriac’s syndrome.7 This syndrome was characterized by unstable diabetes, hepatomegaly, dwarfism, cushingoid features, delayed sexual maturity and hyperlipidemia. Over time, it has been recognized that glycogen accumulation within hepatocytes can be present even without all the other findings described in the Mauriac’s syndrome. This condition have been given many definitions, such as hepatic (or liver) glycogenosis, liver glycogen storage and diabetes mellitus-associated glycogen storage hepatomegaly.2 In the medical literature less than 50 cases of glycogenic hepatopathy are described, but this condition is believed to be extremely underdiagnosed.
Hepatic glycogen levels are regulated by the balance between glycogenesis and glycogenolysis. Mechanistically, glycogenic hepatopathy results from excess accumulation of glycogen in hepatocytes, occurring when a marked or prolonged hyperglycemia is treated with insulin.2,5 The influx of glucose into the hepatocytes is due to passive diffusion independent of insulin levels. Within the cell, glucose is converted into glucose-6-phosphate and transformed to glycogen by the enzyme glycogen synthase. The increase in hepatic glycogen synthesis depends on the presence of a high concentration of glucose and on insulin concentration in the environment. The enzyme glycogen synthase is converted from an inactive (phosphorylated) form into the active (dephosphorylated) form by a phosphatase, and phosphatase concentration is maintained by insulin and relies on the presence of glucose.8 Blood glucose and insulin levels often demonstrate fluctuations in diabetic patient with poor metabolic control and these fluctuations promote hepatic glycogen accumulation. Thus, glycogenosis is the consequence of the combined effect of hyperglycemia (which promotes the flow of glucose into the hepatocytes) and hyperinsulinemia (which promotes the conversion of glucose to glycogen). The literature suggests that glycogen hepatopathy is reversible with improved glycaemic control but liver damage may typically recur, as in this case.3,4
In the patient described in this report, differential diagnosis included several other possible causes of liver damage for both her young age and her previous medical history. NAFLD could be hypothesized for the presence of dyslipidemia, mild overweight, and diabetes.9 This possibility was supported by a bright liver appearance at ultrasound and was considered the main cause of damage during the first hospital admission. However, NAFLD rarely causes aminotransferase elevations similar to the ones observed in this patient, and acute appearance and disappearance of biochemical abnormalities is uncommon. Nonetheless, some degree of fatty infiltration was observed when a biopsy was performed, and its contribution to liver damage is possible. Nonetheless, this case confirms the important point that glycogenic hepatopathy cannot be distinguished from NAFLD clinically or by ultrasound, and always requires a high degree of clinical suspicion and a liver biopsy.2
The presence of other autoimmune diseases, including type 1 diabetes and thyroiditis, the young age of the patient, female gender, and positive antinuclear antibodies (albeit at low titer) suggested autoimmune hepatitis as a possible cause of injury. Nonetheless, biopsy showed the absence of any inflammatory infiltrate, no hypergammaglobulinemia was present and the AIH score was ultimately low.10 Liver disease associated with celiac disease could be considered as an additional cause of liver enzyme elevation, particularly in the light of the poor adherence to gluten-free diet. However, this condition usually causes mild aminotransferase elevation and histology usually shows a non-specific chronic hepatitis pattern or a normal liver.11
In conclusion, we report a case of glycogenic hepatopathy associated with type 1 diabetes, causing a clinical picture of relapsing hepatitis. Diagnosis was not reached at first because better adherence to antidiabetic treatment rapidly caused normalization of the tests and biopsy, the only way to diagnose glycogenic hepatopathy, was performed only when the clinical syndrome recurred. This report highlights the need to consider this usually underdiagnosed entity when dealing with patients with type 1 diabetes and signs of liver injury.
AcknowledgmentWe are indebted to Professor Amar P. Dhillon for reviewing the case and for helpful discussion.