HFE-related Hemochromatosis (HH) is characterized by marked phenotype heterogeneity, probably due to the combined action of acquired and genetic factors. Among them, GNPAT rs11558492 was proposed as genetic modifier of iron status, but results are still controversial. To shed light on these discrepancies, we genotyped 298 Italian p.C282Y homozygotes and 169 healthy controls.
Material and MethodsAllele and genotype frequencies were analysed and compared with those reported in Exome Variant Server (EVS). To explore the role of rs11558492 as a potential modifier of iron status, serum ferritin (SF), liver iron concentration (LIC) and iron removed (IR) were studied according to allele and genotype frequencies. In addition, the effect of the SNP on liver fibrosis was examined comparing patients with absent/mild-moderate fibrosis to those with severe fibrosis-cirrho-sis.
ResultsGNPAT rs11558492 minor allele (G) frequency (MAF) was 20.3% in HFE-HH, 17.2% in controls and 20.6% in EVS database. Genotype frequencies were 64% and 69.2% (AA), 31.2% and 27.2% (AG), 4.8% and 3.6% (GG) in HFE-HH and controls, respectively. No significant differences were found comparing genotype and allele frequencies even selecting subgroups of only-males with extreme phenotypes and low alcohol intake. SF, IR and LIC levels did not significantly differ according to rs11558492 genotypes. Also, MAF did not differ between patients with absent/mild fibrosis and severe fibrosis/cirrhosis.
ConclusionsOur findings indicate that GNPAT rs11558492 is not a major modifier of iron status and is not associated with liver fibrosis in HFE-HH patients.
HFE-related Hemochromatosis (HH) is the most common inherited iron overload disease in Caucasian populations. Homozygosity for the p.C282Y mutation in HFE is the typical defect conferring a strong predisposition to develop the disease.1 It leads to inadequate production of hepcidin, the master regulator of iron homeostasis through a still incompletely understood mechanism.1 Several evidences indicate that p.C282Y homozygosity has variable penetrance and expression. While most homozy-gotes for the p.C282Y mutation show alteration of serum iron indices, severe iron overload and iron-related complications are observed in less than 30% of men and 5% of women.2-4 Alcohol intake and obesity, and coexistence with other diseases able to increase iron absorption or favour liver damage, can influence phenotype expression.5-9 The role of genetic modifiers was demonstrated in HFE-knock-out mice,10 but association studies between genetic markers and disease phenotype in humans have given conflicting results.11,16 Recently, McLaren, et al.17 identified glyceronephosphate O-acyltransferase (GNPAT) rs11558492 A > G polymorphism (p.D519G) as a new potential genetic modifier of HFE hemochromatosis pheno-type in p.C282Y homozygotes. The prognostic role of this polymorphism has been evaluated in other cohorts,18-20 but none demonstrated an association between the SNP and markers of iron overload. However, Besson-Fournier, et al. found allele G to be significantly enriched in the subset of males with markedly increased iron stores. More recently, in a small cohort of healthy women and in 59 individuals not carrying HFE genotypes 8 hours after iron challenge an association between minor (G) GNPAT al-lele and serum iron was reported.21,22 To contribute in understanding the role of this SNP as a possible modifier of iron phenotype, we analysed alleles and genotypes frequencies and their relationship with iron parameters in a cohort of 298 HFE p.C282Y homozygotes of Italian ancestry and a group of 169 male healthy blood donors.
Material and MethodsPatientsTwo hundred and ninety-eight patients (205 men and 93 women) homozygous for p.C282Y in HFE with a median age of 48 years (interquartile range (IQR) 36-57 years) were studied. They attended the Centre for Disorder of Iron Metabolism at ASST-Monza-S.Gerardo Hospital, Fondazione IRCCS Ca’ Granda Policlinico Hospital in Milan and Policlinico GB Rossi in Verona. Patients were selected based on availability of serum ferritin (SF), trans-ferrin saturation (TS), alcohol intake and serum alanine aminotransferase (ALT) at diagnosis, and good quality DNA. Patients with coexistent chronic liver diseases (al-pha-1 antitrypsin deficiency, autoimmune hepatitis, chronic B or C virus hepatitis), and alcohol intake > 50 g/day in men and > 30 g/day in women23 were excluded.
Two-hundred and twenty-six patients (160 men and 66 women) underwent to liver biopsy for diagnostic (before HFE testing) and/or prognostic aim. Liver fibrosis was assessed according to Ishak, et al.24 Liver iron concentration (LIC) was available in 191 (64%) patients and was measured on liver biopsy samples in fresh or deparaffinized specimens (N = 155) or by quantitative magnetic resonance (MR) as previously reported.25,26 The lower number of available LIC is explained by the fact that:
- •
Biopsy samples were not always as long as needed to allow adequate LIC measurement.25
- •
MR iron quantitation was implemented only recently n our centres.26
Iron removed (IR) after iron depletion was available in 234 (78.5%) patients and measured as previously reported.23
One hundred and sixty-nine Italian health blood donors, selected from a cohort previously studied to define revalence of HFE mutations in Italy,27 were also evaluated as controls. Selection was based on HFE genotype (only wild-type or p.H63D heterozygotes were retained), sex (females were excluded), availability of haemoglobin and SF at first blood donation, and good quality DNA.
Patients gave their informed consent for liver biopsy and both patients and controls for using their DNAs previously collected for HFE testing according to Institutions’ policy. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the appropriate institutional review committee.
GNPAT polymorphism analysisGenomic DNA of patients and controls was extracted from peripheral blood leucocytes using the Wizard® Genomic DNA Purification kit (Promega, Madison, WI, USA) or the ZR DNA-Card Extraction Kit™ (Zymo Research, Irvine, CA, USA) according to manufacturer's instructions. GNPAT rs11558492 genotyping was carried out by tetra-primer Amplification Refractory Mutation System (ARMS-PCR) and verified by 1.5% of agarose gel stained with ethidium bromide, and/or TaqMan® 5’-nuclease assays (LifeTechnologies, Carlsbad, CA). Random samples were confirmed by direct sequencing.
Statistical analysisMedian and IQR were used for descriptive purposes as appropriate. Hardy-Weinberg equilibrium was examined for all subjects using χ2 test. To evaluate the prognostic role of GNPAT polymorphism, we evaluated allele and genotype frequencies according to TS, SF, LIC and IR levels in patients and SF levels in controls. In patients, analyses were performed in the whole series and in males only. A further analysis was done comparing subgroups of male patients with extreme phenotypes: severe iron overload (SF > 2,000 μg/L, IR > 10 g and/or LIC > 250 μmol/ g, alcohol intake < 30 g/day), mild iron overload (SF < 1000 μg/L, IR < 5 g and/or LIC < 100 μmol/g, alcohol intake < 30 g/day). To evaluate the effect of GNPAT rs11558492 on liver fibrosis we grouped patients in two groups: those with absent or mild-moderate fibrosis (stage 0-3) and with severe fibrosis-cirrhosis (stage 4-6) as previously reported.23 Comparisons of GNPAT rs11558492 al-lele and genotype frequencies between groups were done by Fisher's exact test. Statistical analyses were performed using GraphPad Prism software, version 4.0 (San Diego, CA, USA). P-value < 0.05 was the accepted level of significance.
ResultsGenotype distributions of GNPAT rs11558492 were in Hardy-Weinberg equilibrium in patients and controls. Table 1 shows main data of HFE-HH patients and controls. Serum ferritin significantly correlated with LIC (R2 = 0.42; p < 0.0001) and IR (R2 = 0.6; p < 0.0001), and LIC with IR (R2 = 0.51; p < 0.0001). As shown in table 2, genotype and allele frequencies were comparable in patients and healthy blood donors, and those reported in the Exome Variant Server (EVS) database (genotypes: AA = 64%; AG = 31.2%; GG = 4.8%; alleles: A = 79.7% and G = 20.3%).
Main data of HFE-HH patients and controls. Data are expressed as median (interquartile range).
| Variable | HFE-HH (N = 298) | Healthy blood donors (N = 169) |
|---|---|---|
| Males | 205 | 169 |
| Age (years) | 48 (36-57) | 37 (31-48) |
| Transferrin saturation (%) | 86 (71-95.5) | NA |
| Serum ferritin (mcg/L) | 1000 (571-1828.5) | 79 (54-116) |
| Iron removed† (g) | 6.6 (4-10) | - |
| Liver iron concentration (umol/g)†† | 218.6 (167.2-341.8) | - |
| Alanine aminotransferase (U/L) | 40 (23-65) | NA |
Genotype and allele frequencies (number and percentage) of GNPAT rs11558492 polymorphism in HFE-HH patients (whole cohort and male-only) and healthy blood donors. Data are expressed as numbers and (percentages)
| HFE- HH (N = 298) | HFE- HH male-only (N = 205) | Healthy blood donors (N = 169) | |
|---|---|---|---|
| Genotypes | |||
| AA | 191 (64) | 128 (62.4) | 117 (69.2) |
| AG | 93 (31.2) | 67 (32.6) | 46 (27.2) |
| GG Alleles | 14 (4.8) | 10 (5.0) | 6 (3.6) |
| A | 475 (79.7) | 323 (78.7) | 280 (82.8) |
| G | 121 (20.3) | 87 (21.3) | 58 (17.2) |
No significant difference between patients and controls.
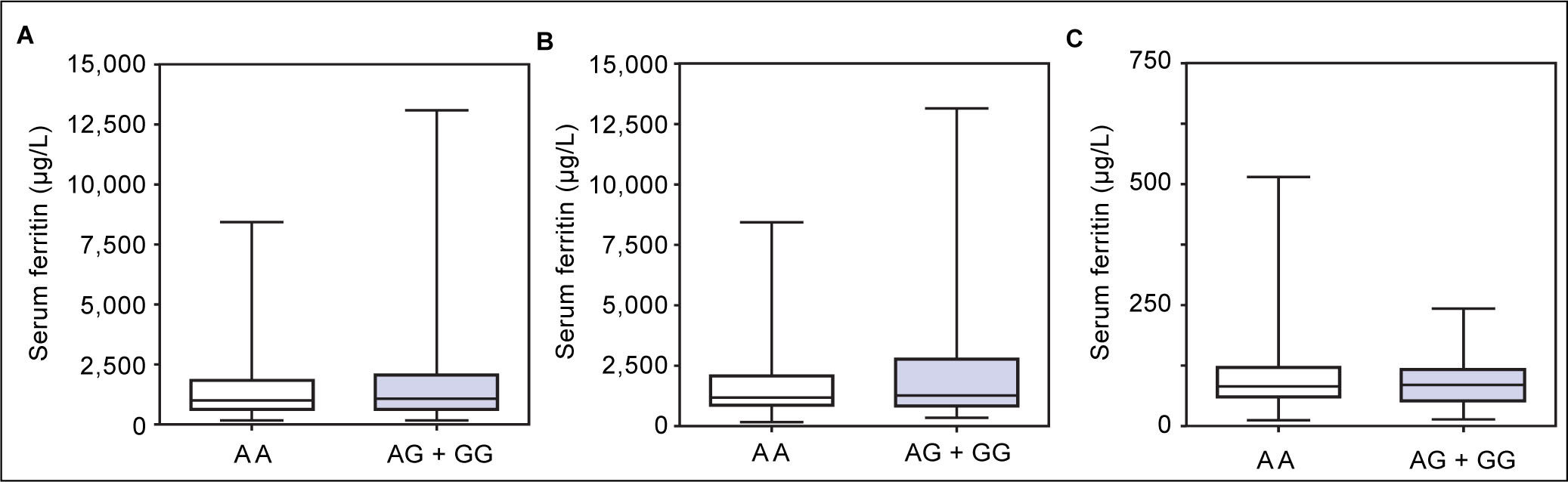
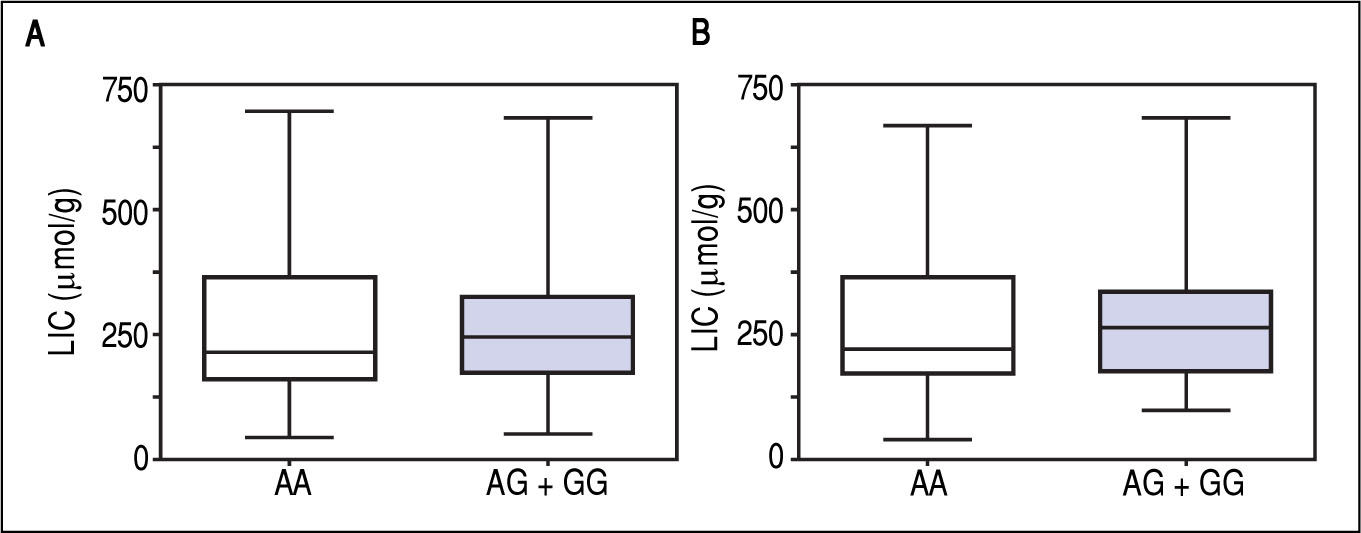
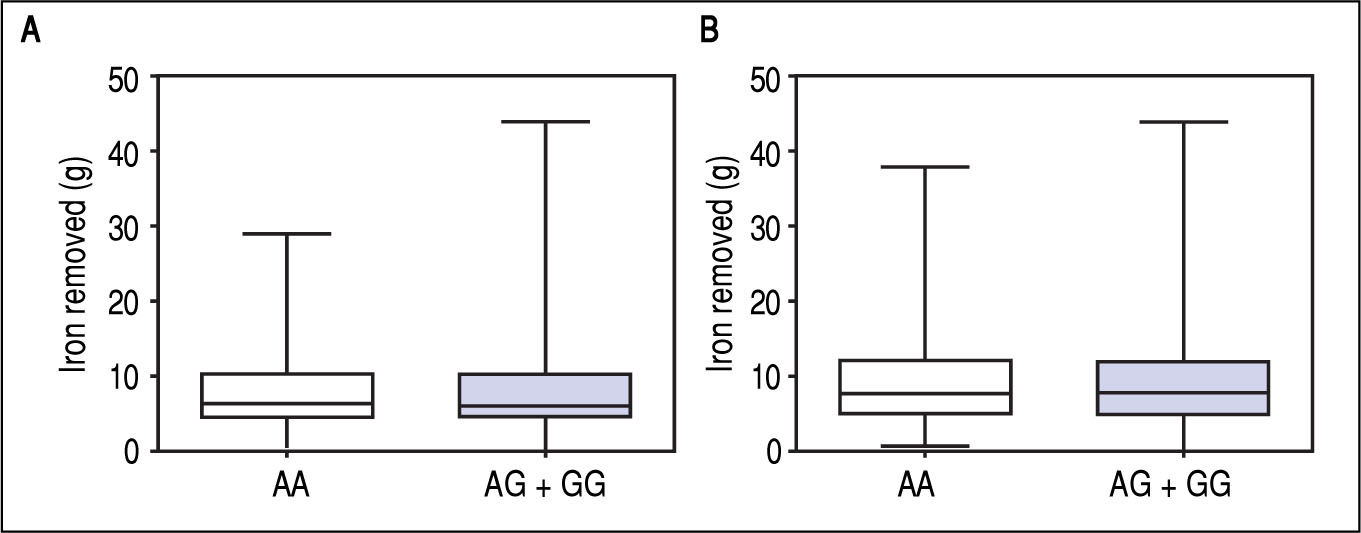
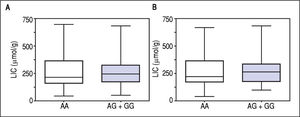
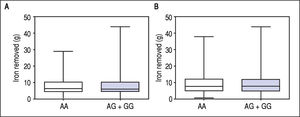
Figure 1 A-C shows median and IQR of SF according to GNPAT genotypes in the whole cohort [AA: 1,000 μg/L (552-1815.5); AG + GG: 1061 μg/L (610.5-1946.5)] and male-only [AA: 1145 μg/L (801-2019); AG + GG: 1245 μg/L (771-2721)] HH patients, and in controls [AA: 78.0 μg/L (55.5-117.5); AG + GG: 83.0 μg/L (53-110)]. No significant difference was observed. Accordingly, also LIC and IR did not differ according to GNPAT genotypes both in the whole cohort of HH patients [LIC: AA: 214.2 μmol/g (158.7-361.2); AG + GG: 242.6 μmol/g (167.2-310.2); IR: AA: 6.25 g (4-9.9); AG + GG: 5.77 g (3.9-10)] and in male-only subgroup [LIC: AA: 220 μmol/g (167.2-365.1); AG + GG: 263.1 μmol/g (179.4-320); IR: AA: 8 g (5-12); AG + GG: 8 g (4.7-11.7)] (Figures 2 A-B and 3 A-B). No differences were also observed for TS [AA genotype: 85% (72-91.6); AG + GG genotypes: 90% (76.7-97.5)], haemoglobin and ALT (data not shown). We then measured the frequency of GNPAT alleles and genotypes in the subgroups of patients with extreme phenotypes. Thirty and thirty-eight men fulfilled the criteria for severe and mild iron overload, respectively. Allele and genotype frequencies did not differ between the two subgroups (Table 3) and compared to EVS database. Fifty-eight out of 226 patients had severe fibrosis/cirrhosis. Minor allele frequency (MAF) was slightly higher in patients with severe fibrosis/cirrhosis (25%) than in those with absent/mild fibrosis (20.5%), but not significantly so (p = 0.36). Similarly, in the subgroup of men with alcohol intake < 30 g/day, MAF was 25% vs. 19.1%, respectively (p = 0.31).
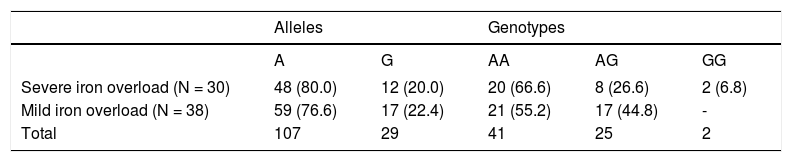
Allele and genotype frequencies in men with HH selected according to iron phenotype. Data are expressed as numbers and (percentages).
| Alleles | Genotypes | ||||
|---|---|---|---|---|---|
| A | G | AA | AG | GG | |
| Severe iron overload (N = 30) | 48 (80.0) | 12 (20.0) | 20 (66.6) | 8 (26.6) | 2 (6.8) |
| Mild iron overload (N = 38) | 59 (76.6) | 17 (22.4) | 21 (55.2) | 17 (44.8) | - |
| Total | 107 | 29 | 41 | 25 | 2 |
Severe iron overload: SF > 2000 μg/L, IR >10 g and/or LIC > 250 μmol/g, alcohol intake < 30 g/day. Mild iron overload: SF < 1,000 μg/L, IR < 5 g and/or LIC < 100 μmol/g, alcohol intake < 30 g/day).
We evaluated the frequency of GNPAT alleles and genotypes in a cohort of Italian p.C282Y homozygous patients and healthy blood donors and the relation between the SNP and markers of iron status. The frequency of alleles and genotypes in patients did not differ compared to healthy blood donors and those reported in EVS database. Allele frequencies of GNPAT p.D519G were slightly lower than that reported in other series of HH patients originating from Western Europe,18-20 but similar to that of European Americans from the EVS database. Also, we did not find associations between alleles and genotypes and TS, SF, LIC and IR in HFE-HH patients, and SF in blood donors. Our results contrast those reported by McLaren, et al.17 in North-American and Australian HH patients, but agree to those reported in other cohorts18,19,28 of HFE-HH patients. Also, we could not find differences comparing al-lele and genotype frequencies in the subsets of HFE-HH patients selected for mild and severe iron phenotypes. We used restrictive criteria (SF > 2,000 μg/L and IR > 10 g and/or LIC > 250 μmol/g) to define severe iron pheno-type in HFE-HH, but similar results were observed even using a ferritin cut-off of 1,000 μg/L instead of 2,000 μg/L (N = 67 men with alcohol intake < 30 g/day; MAF: 23.1%).
McLaren, et al.17 linked controversies to the design and power of studies and to inclusion criteria and supported the role of GNPAT as a modifier of iron phenotype showing that it might participate in hepcidin regulation through a still undefined mechanism in vitro. We hypothesised that analysing subjects of Italian ancestry whose genetic structure is heterogeneous might help in shedding light on these discrepancies.29 Our findings indicate that GNPAT rs11558492 is not a major modifier of iron status in HFE-HH, although we cannot exclude that it could be part of a more complex genetic background that might include more than one mild modifier.11,16 Accordingly, we were not able to show any relation between GNPAT polymorphism and iron status in healthy blood donors. The latter finding contrast those by Hsiao, et al.21 who showed an association between serum iron at baseline and after an iron test in 83 young women. Similarly, Rametta, et al.22 demonstrated that carriers of the G variant without HFE genotypes at risk (p.C282Y wild type or p.H63D heterozygotes) have mildly increased iron absorption 8 hours after ferrous sulphate challenge. However, both studies did not find association between GNPAT and SF that represents the best marker of iron stores in healthy people. These results raised further questions on the clinical relevance of GNPAT as iron modifier. Last, GNPAT rs1155849 was not associated with severe fibrosis/cirrho-sis, confirming previous results.18 In conclusion, several evidence indicate that homozygosity for p.C282Y in HFE is necessary but not sufficient to develop significant iron overload. There is general agreement that acquired factors, such as alcohol intake, hepatic steatosis, obesity and coexistence of chronic viral hepatitis, are major modifiers of iron or clinical phenotype in HH.5.9 Conversely, though milder effects cannot be ruled out in the absence of adequately powered cohorts, no polymorphisms that came up from studies done up to now seem to exert a major effect on iron phenotype to be considered for defining genetic susceptibility to fully penetrant HFE-HH in clinical practice. Interestingly, two recent studies strongly suggested a role for rs236918 in PCSK7 gene as a modifier of risk of liver fibrosis in p.C282Y homozygotes.23,30 Exome or whole-genome sequencing studies by next-generation sequencing technologies will enable to further explore if there exist some other unknown genetic variants with clinical significance in HFE-HH patients carefully selected and controlled for environmental factors.
Abbreviations- •
ALT: Serum alanine aminotransferase.
- •
ARMS-PCR: Amplification refractory mutation system – polymerase chain reaction.
- •
EVS: Exome variant server.
- •
GNPAT: Glyceronephosphate O-acyltransferase.
- •
HH: Hereditary hemochromatosis
- •
IQR: Interquartile range.
- •
IR: Iron removed.
- •
SF: Serum ferritin.
- •
SNP: Single nucleotide polymorphism.
- •
TS: Transferrin saturation.
The authors declare no conflict of interest.
Financial SupportThis work was partially supported by: Associazione per lo studio dell’Emocromatosi e delle Malattie da Sovracca-rico ONLUS +Fe, Monza, Italy; Ricerca Corrente Fon-dazione IRCCS Ca’ Granda Policlinico Milano, Italy; Associazione Malattie Metaboliche del Fegato ONLUS, Milano, Italy and Bando Medicina Molecolare 2014 Fon-dazione IRCCS Ca’ Granda Policlinico – Istituto Nazion-ale di Genetica Molecolare, Milan, Italy.