Herbal hepatotoxicity is a rare and poorly described disease because reported cases are mostly scattered and lack an appropriate causality assessment. We now describe in detail the clinical picture of herbal hepatotoxicity by extracts of Greater Celandine (GC), syn. Chelidonium majus L. from the Papaveraceae family, which contain more than 20 ingredients including various biologically active isoquinoline alkaloids. For this purpose, we analyzed and reviewed published cases of 16 patients from various European countries. In all patients, herbal hepatotoxicity was of probable and highly probable causality for GC, using the original and updated scale of CIOMS (Council for International Organizations of Medical Sciences). GC associated hepatotoxicity usually has an acute clinical course exhibiting a hepatocellular pattern of injury and is correlated to an idiosyncratic reaction with its metabolic subtype. Jaundice combined with high values of serum aminotransferases was present in virtually all cases with favourable outcome despite severe clinical course. In conclusion, GC hepatotoxicity is a typical herbal hepatotoxicity with a sound causality track for GC, but there is uncertainty regarding the respective causative compound(s). The present detailed review of GC hepatotoxicity may serve as an example for clinical causality assessments of future cases of liver injury due to other herbs.
Herbal hepatotoxicity or herb induced liver injury (HILI) is a disease with multiple facets to be considered for clinical case evaluation and disease characterization.1,2 In general, however, detailed case descriptions of liver injury caused by a single herb or herbal product are rare, because reports with few cases prevail and numerous variables confound. In most of the reported HILI cases there is also lack of an appropriate causality assessment. To cope with these issues, the best approach to characterize herbal hepatotoxicity by one single herb is to collect hepatotoxicity cases and to analyze the data of case reports and spontaneous reports step by step, using also an appropriate diagnostic causality algorithm.
Greater Celandine (GC), syn. Chelidonium majus L. from the Papaveraceae family, is a herb whose sap tastes fetid, thereby disliked by animals, but collected by humans to prepare medicinal herbal extracts. GC increases biliary flow in experimental studies3 and was approved by the Commission E of the German regulatory agency BfArM (Bundesinstitut für Arzneimittel und Medizinprodukte, Bonn) as herbal drug for spastic discomfort of the gastrointestinal tract including the bile ducts.4,5 Spontaneous case reports in Germany5 and published case reports in European countries6-15 including Germany,6-8,10,11 the Netherlands,9 Belgium,12 and Italy13-15 suggested that the use of GC might carry the risk of liver injury. Consequently, detailed analyses and causality assessments have been performed in all cases of primarily assumed GC hepatotoxicity with respect to both published case reports16 and spontaneous reports.17
This review summarizes the evidence for the hepatotoxic potential of GC and describes clinical features of this particular HILI based on 16 European cases of GC hepatotoxicity with a highly probable or probable causality level. The description of thorough investigations associated with the evaluation of the GC cases may have impact on future evaluations of HILI cases by other herbal drugs and herbal supplements.
Published Case Reports and Spontaneous ReportsGC hepatotoxicity has primarily been assumed in a total of 69 cases, with 21 cases published as case reports since 1998,6-15 and 48 cases as spontaneous reports communicated to the German regulatory agency BfArM.5 Of these 48 spontaneous reports, the regulatory agency judged four cases as not sufficiently documented cases, 14 cases as well documented ones, and the remaining 30 cases as poorly documented cases, albeit sufficient for causality assessment. There were also 6 case reports that were included in the list of spontaneous reports and represented case duplicates. Overall, the regulatory agency assessed 23 cases with more or less details and judged one as being unrelated to GC intake and 22 cases with a probable or possible causality.5 The 21 published cases plus 22 regulatory cases represented a total of 43 patients and have been submitted to further evaluation including causality assessment for GC intake.16,17
Assessmnt of these 43 patients with primarily assumed GC hepatotoxicity uncovered numerous confounding factors. The major problem was lack of consideration of possible alternative explanations, mostly from comedication but also from preexisting biliary diseases.16,17 These included symptomatic biliary stones and cholangitis in the course of biliary tract infection; cystic bile duct obstruction; symptomatic cholecystolithiasis; cholecystitis; cholecystitis with microcalculi in the gallbladder; choledocholithiasis requiring endoscopic sphincterotomy and endobiliary stenting, and diffuse bowel inflammation; extrahepatic bile duct obstruction due to excessive hilar adenopathy; autoimmune hepatitis (AIH); drug induced liver injury (DILI); HILI by other herbal extracts; adenovirus hepatitis; hepatitis with Mallory bodies; and pancreatitis.16,17 Alternative explanations in this setting are not unusual and have also been established in other cases of primarily suspected HILI and DILI.18
Striking differences in data quality existed between published case reports16 and spontaneous reports.17 In particular, product information and treatment modalities were poorly documented in the case reports16 but satisfactory in the spontaneous reports.17 Exclusion of HAV, HBV, and HCV infections was provided in all published case reports16 but not stringent in the spontaneous reports.17 In both groups, major shortcomings were also evident in documentation of diagnostic criteria.16,17 Similar problems of data presentation have been noticed in other studies.18-23
Causality AssessmentTo establish causation for GC in these cases of primarily suspected GC hepatotoxicity,5-15 a diagnostic algorithm consisting of the liver specific, for hepatotoxicity validated, structured, quantitative and updated scale of CIOMS (Council for International Organizations of Medical Sciences),24-26 has been applied.16,17 Among the 21 case reports and the 22 spontaneous reports, causality for GC was judged to be possible, unlikely, or excluded in a total of 27 cases.16,17 In published case reports causality for GC was highly probable in 2 cases and probable in 6 cases,16 with identical results for the evaluated spontaneous reports.17 Combining the results of the case reports with those of the spontaneous reports, causality for GC was finally highly probable in 4 cases and probable in 12 cases. Overall results were identical whether the original or the updated CIOMS scale was used.16,17 These 16 cases with a highly probable or probable causality for GC are used to characterize GC hepatotoxicity as a distinct liver disease and are designed as the study group.
Clinical CharacteristicsDetails of the 16 cases to be considered for the disease characterization are presented for the 8 published case reports as cases 1-8 and for the 8 spontaneous reports as cases 9-16 (Table 1).
Clinical data of all 16 patients presented by published case reports (cases 01-08) and spontaneous reports (cases 09-16) with liver disease in highly probableand probable causal association with the treatment by Greater Celandine (GC).
| Patient (Identification) | Specific information for each individual patient |
|---|---|
| 1(Strahl et al., 19986 42 years, female) | GC extract of known brand name and manufacturer (3 capsules/d containing each 200 mg of probably dried herb for 9 months). Bloating as indication for treatment. Latency period of 2 months for first symptoms of itching and jaundice, at rechallenge 1 month. ALT 755 U/L, AST 350 U/L, ALP 221 U/L. Upon cessation of GC treatment, rapid decrease but not normalization of ALT values reported. Readministration of GC with positive result. Exclusion of virus hepatitis A-C and infections of other hepatotropic viruses reported, with lack of any details regarding hepatitis A, B, and C, CMV, EBV, HSV or VZV. Exclusion of biliary obstruction by sonography. Exclusion of autoimmune hepatitis reported with lack of specified parameters. Normal values of iron and copper parameters. Liver histology: Acute hepatitis with confluent liver cell necroses and little inflammation.Final diagnosis: highly probable GC hepatotoxicity. |
| 2(Benninger et al., 1999,8 their case 5, 37 years, female) | GC extract as drug of known brand name and manufacturer (unknown dose/d for 3 months). Atopic eczema as indication for GC treatment. Various herbal and homeopathic drugs as CD. Latency period of 3 months until symptoms of nausea and jaundice. ALT 813 U/L, AST 898 U/L, ALP 249 U/L. ALT course described. Positive reexposure test.for GC. Five months after GC discontinuation, normalization of liver parameters reported. Exclusion of infections by HAV, HBV, HBC, HEV, CMV and EBV. SMA 1:40, exclusion of AIH. Ultrasound examination with normal bile ducts. Liver histology not done.Final diagnosis: Highly probable GC hepatotoxicity. |
| 3(Benninger et al., 1999,8 their case 6, 65 years, female) | GC extract of unknown brand name and manufacturer (unknown dose/d for 3 months). Dyspepsia as indication for GC treatment. Latency period and symptoms not recorded. No CD. ALT 152 U/L, AST 89 U/L, ALP 451 U/L. After GC discontinuation, ALT course not sufficiently documented. Normalization of liver values 3 months after GC withdrawal. Exclusion of common causes for hepatitis reported, but lack of information regarding specific parameters. Lack of ultrasound data. Liver histology: Moderate drug induced hepatitis with low grade single cell necrosis.Final diagnosis: Probable GC hepatotoxicity. |
| 4(Crijns et al., 2002,9 42 years, female) | Herbal mixture of GC and curcuma root (Curcuma tonga rhizoma) of known brand name and manufacturer (unknown dose/d for 2 months). Not further described skin complaints as indication for treatment. Before admission, paracetamol (500 mg) tablet on one day. Latency period: 5 weeks until jaundice. Fever 40.5 °C for 2 weeks, starting 2 weeks after initiation of GC treatment. ALT 1490 U/L, AST 838 U/L, ALP 265 U/L. Following cessation of the herbal mixture, ALT course described with normalization of liver values after 2 months. Exclusion of acute hepatitis A-C and infections by CMV, and EBV, but HSV and VZV not assessed. Normal titres of ANA, but no data of AMA, SMA, and LKM. Sonography with normal biliary tract. Liver histology: Severe acute hepatitis of viral or toxic drug cause.Final diagnoses: Probable GC hepatotoxicity and possible curcuma hepatotoxicity. |
| 5(Stickel et al., 2003,10 their case 2, 69 years, male) | GC extract as drug of known brand name and manufacturer (80 capsules within 5-6 weeks). Postprandial abdominal discomfort as indication for GC treatment. Latency period of 5-6 weeks until symptoms of weakness, abdominal pain in the right upper quadrant, nausea, jaundice, and dark brown urine. Medical history included cholecystectomy 4 years ago. Lack of regular comedication. Alcohol consumption below 20 g/d. ALT 881 U/L, AST 466 U/L, ALP 312 U/L. ALT course not recorded. Exclusion of acute viral hepatitis including HAV, HBV, HCV, CMV, EBV. Autoimmune parameters not assessed. By abdominal ultrasound and magnetic resonance tomography common and intrahepatic bile ducts inapparent. Liver histology: Cholestatic hepatitis compatible with drug toxicity.Final diagnosis: Probable GC hepatotoxicity. |
| 6(Rifai et al., 2006,11 58 years, male) | GC extract as drug of known brand name and manufacturer (unknown amounts of tablets/d for 3 weeks). Biliary spasms as indication for GC treatment. Latency period: of 3 weeks until fatigue, dark urine, itching, jaundice, and pale stool. No CD. ALT 903 U/L, AST 380 U/L, ALP 516 U/L. After GC withdrawal well documented ALT course with ALT normalization within 4 weeks. Well documented exclusion of hepatitis A-C, and E, and of infections by CMV, EBV, HSV, and VZV reported. Well documented exclusion of infectious, autoimmune, metabolic, and genetic causes of acute hepatitis. Sonography with slightly thickening of the gall bladder and otherwise normal biliary tract. Liver histology: Lobular hepatitis with severe cholestasis and moderate inflammation that included also the bile ducts.Final diagnosis: Probable GC hepatotoxicity, but also possible causality for biliary disease. |
| 7(Conti, et al., 2008,13 46 years, female) | GC extract as solution of known brand name and manufacturer, containing also other herbs as are Lycopodium serrata, Carduus marianus, Hamamelis, Ruta, Sepia, Pulsatilla, Collinsonia, and Hydrastis (50 drops/d for 8 weeks). Insomnia and for sedation as indication for treatment. Latency period of 8 weeks until symptoms of nausea, anorexia, asthenia, and abdominal discomfort. Herbal mixture with various herbs and the potentially hepatotoxic Lycopodium serrata as CD. ALT 2,364 U/L, AST 737 U/L, ALP 255 U/L. Rapid decrease of ALT in the further course following treatment cessation with normalization after 2 months. Exclusion of HAV, HBV, HCV, CMV, EBV, and HSV. Specified serological tests for autoimmune diseases negative. Sonography without reported biliary tract abnormalities. Liver histology: Moderate mixed inflammatory infiltrate with eosinophils.Final diagnoses: Probable GC hepatotoxicity, probable Lycopodium serratum hepatotoxicity. |
| 8(Moro et al., 2009,14 65 years, male) | GC extract as herbal tea derived from GC leaves (1 cup/d for 1 month). Pyrosis as indication for GC treatment. Lansoprazole 15 mg/d for 2 years as current CD. Latency period of 1 month. Asthenia, dyspepsia, dark urine, and jaundice as symptoms. ALT 4,765 U/L, AST 3,235 U/L, ALP not reported. ALT course not reported, but normalization of all liver parameters within 2 months. Three months before symptom onset, treatment with clarithromycin and amoxicillin for 1 week. All antibodies for not further specified hepatic viruses resulted negative except for anti-HCV that was found positive despite negative HCV-PCR. Autoimmune parameters not reported. Hepatomegaly by ultrasound examination. Liver histology: Moderate drug induced hepatitis.Final diagnosis: Probable GC hepatotoxicity. |
| 9(BfArM 95003848 32 years, male) | GC extract as drug of known brand name and manufacturer (2 capsules/d for not clearly defined duration). Upper abdominal pains as indication for treatment. Latency period not reported, jaundice as symptom. ALT 2,196 U/L, AST 714 U/L, ALP 256 U/L. Upon cessation of GC treatment, decrease but not normalization of ALT and AST values, with lack of reported ALP value. Readministration of GC with pruritus and not further specified increases of liver values and lack of complete resolution upon dechallenge. Overall course of ALT not sufficiently documented, neither at first and second dechallenge, nor in the interval and after the second challenge. Undulating ALT values of unknown clinical significance. Exclusion of virus hepatitis reported, with lack of any details regarding hepatitis A, B, and C, CMV, EBV, HSV or VZV. Exclusion of biliary obstruction by sonography and ERCP. Exclusion of autoimmune hepatitis with lack of reported parameters. Normal values of ceruloplasmin, α-1 Antitrypsin, and electrophoresis. Liver histology: Unspecific hepatitis with liver cell necroses. Poorly documented case including questionable rechallenge and lack of ALT normalization.Final diagnosis: Probable GC hepatotoxicity. |
| 10(BfArM 96026841 55 years, female) | GC extract as drug of known brand name and manufacturer (3 capsules/d for 6 weeks). Upper abdominal pains as indication for treatment. Latency period of 6 weeks with jaundice as symptom. Diltiazem 90 for several years and doxycycline for 10 days (start prior to jaundice) for treatment of erythema migrans as CD. ALT 2,016 U/L, AST 620 U/L, ALP 398 U/L. After cessation of GC treatment, normalization of ALT not reported and with 201 U/L on day 19 still increased. Overall ALT course poorly documented. Exclusion of hepatitis A, B, and C reported without details of assessed parameters. Lack of exclusion of virus infections by CMV, EBV, HSV, and VZV. Negative results for AMA, SMA, LKM, and actin. Exclusion of biliary obstruction by sonography and ERCP. Liver histology: Compatible with drug induced liver injury.Final diagnosis: Highly probable GC hepatotoxicity. |
| 11(BfArM 98000501 65 years, male) | GC extract as drug of known brand name and manufacturer (2-3 capsules/d for 42 days). To increase bile flow after cholecystectomy 20 years ago as indication for treatment. Latency period of 42 days with itching and jaundice as symptoms. ALT 461 U/L, AST 355 U/L, ALP 260 U/L, normalization not reported. After GC discontinuation, on day 12 ALT 235 U/L. Exclusion of hepatitis A-C and of infections by CMV, EBV, HSV, and VZV. AMA negative, exclusion of autoimmune hepatitis reported but individual parameters not described. Upon sonography and ERCP normal bile ducts after cholecystectomy.Final diagnosis: Highly probable GC hepatotoxicity. |
| 12(BfArM 98001447 49 years, female) | GC extract as drug of known brand name and manufacturer (3 tablets/d for 4 weeks). Upper abdominal pains as indication for treatment. Latency period of 3.5 weeks with reduced appetite, bloating, epigastric pain, nausea, vomiting, and jaundice as symptoms. ALT 2928 U/L, AST 1116 U/L, ALP 408 U/L. After GC discontinuation, on day 7 ALT was 1356 U/L, and on day 20 it was 426 U/L. Normalization of ALT has not been reported. Exclusion of hepatitis A-C, E and F, and of infections by CMV, EBV, and HSV, but not of VZV. Normal values of ANA, AMA, and SMA. Upon ultrasound and ERC, normal bile ducts and cholecystolithiasis or cholesterol polyps of the gallbladder, by ultrasound questionable cholecystitis. Liver histology: Severe portal hepatitis with beginning fibrosis.Final diagnosis: Probable GC hepatotoxicity. |
| 13(BfArM 98001607 59 years, female) | GC extract as drug of known brand name and manufacturer (3 tablets/d for 7 weeks). Vomiting, upper abdominal pains and gastro-esophageal reflux as indications for treatment. Latency period o 20 days with tiredness, exhaustion, nausea, vomiting, and jaundice as symptoms. Asthma, treated with various sprays, and latent hyperthyroidism without treatment as comorbidities. Maximum values reported for ALT 960 U/L, AST 421 U/L, and ALP 425 U/L, with decrease but not normalization following GC discontinuation, but actual results have not been reported. Through histology, ERCP, and serology (HAV, HBV, HCV) other hepatobiliary diseases excluded, but details not reported. Missing exclusion of infections by CMV, EBV, HSV, and VZV. Poorly documented case.Final diagnosis: Probable GC hepatotoxicity. |
| 14(BfArM 98008527 60 years, female) | GC extract as drug of known name and manufacturer (3 capsules/d for several weeks). General discomfort as indication for treatment. Latency period of several weeks with abdominal pains, nausea, and jaundice as symptoms. Crataegus extract as CD. ALT 420 U/L, AST 451 U/L, ALP 288 U/L. At discharge after 4 weeks, ALT with 26 U/L still slightly elevated. Exclusion of acute hepatitis A-C and infections by CMV, and EBV, but HSV and VZV not assessed. Normal titres of ANA, AMA, SMA, and LKM. Sonography and ERCP with normal biliary tract. Liver histology: AIH or dug induced liver injury.Final diagnosis: Probable GC hepatotoxicity. |
| 15(BfArM 00000278 65 years, male) | GC extract as drug of known brand name and manufacturer (3 capsules/d for 4 weeks). Bloating as indication for treatment. Latency period of 3.5 weeks with jaundice as symptom. Diclofenac (intermittent), sitosterols, butizide, raubasine, rescinnamine, and reserpine as CD. ALT 950 U/L, AST 570 U/L, normal ALP. Under treatment with cortisone and at discharge, ALT 193 U/L, but normalization of ALT with and without cortisone not reported. Exclusion of hepatitis A-C and of infections by CMV, EBV, and HSV reported. Normal titres of ANA, AMA, and SMA. Sonography and ERCP with normal biliary tract. Liver histology: Hepatitis with cholestasis.Final diagnosis: Probable GC hepatotoxicity. |
| 16(BfArM 02001171 66 years, female) | GC extract as drug of known brand name and manufacturer (0-2 capsules/d for 4.5 months). Dyspepsia as indication for treatment. Latency period of 4.5 months with reduced appetite and jaundice as symptoms. ALT 760 U/L, AST 408 U/L, ALP 337 U/L. On day 14 after GC cessation, ALT 379 U/L, and on day 24 ALT 207 U/L. Normalization of ALT not reported. Exclusion of hepatitis A-C reported, but details not presented. No exclusion of infections by CMV, EBV, HSV, and VZV. Autoimmune parameters not done. Sonography, MRCP and MRT with normal biliary tract. Insufficiency of the mitral valve.Final diagnosis: Probable GC hepatotoxicity. |
Details of relevant clinical data are presented for 16 patients with liver injury by the use of the herb Greater Celandine (GC), compiled on the basis of previous analyses.16,17 In these studies, highly probable and probable causality levels for GC were established in all patients presented as final diagnoses, using the updated CIOMS scale for the individual causality assessment24,25 with identical results obtained also with the original CIOMS scale.26 Half of the patients (cases 01-08) were derived from published case reports,6,8-11,13,14 the other half (cases 09-16) from spontaneous reports of the German regulatory agency BfArM.5 Outcome was favourable in all cases. Upper limits of the normal ranges: ALT 23 and 19 U/L for males and females, respectively; AST 19 and 15 U/L for males and females, respectively; ALP 170 U/L. AIH: autoimmune hepatitis. ALP: alkaline phosphatase. ALT: alanine aminotransferase. AMA: antimitochondrial antibodies. ANA: antinuclear antibodies. AST: aspartate aminotransferase. BfArM: Bundesinstitut für Arzneimittel und Medizinprodukte, Bonn. CD: comedicated drug(s). CIOMS: Council for International Organizations of Medical Sciences. CMV: cytomegalovirus. EBV: Epstein Barr virus. ERCP: endoscopic retrograde cholangiopancreaticography. HAV: hepatitis A virus. HBV: hepatitis B virus. HCV: hepatitis C virus. HSV: herpes simplex virus. LKM: liver kidney microsomal antibodies. MRCP: magnetic resonance cholangiopancreaticography. MRT: magnetic resonance tomography. PCR: polymerase chain reaction. SMA: smooth muscle antibodies. VZV: varicella zoster virus.
The age of the 16 patients of the study group ranged from 32 to 69 years with an average of 55 years (Table 1): 10 female patients with a range from 37 to 66 years (average 52 years), 6 male patients with a range from 32 to 69 years (average 59 years). Thus, the average age was slightly higher in males compared to females. With 13 cases, most of the patients were of German origin (cases 1-3,5,6,9-16), 2 patients originated from Italy (cases 7 and 8), and 1 patient from the Netherlands (case 4).
GC products and comedicationTwelve patients used a GC monopreparation, 2 patients a polyherbal product containing also GC, 1 patient GC leaves for tea, and 1 patient an unknown GC product (Table 1). In 12 out of 16 cases, both brand name and manufacturer were given. There was no information as to whether these 12 GC monopreparations or 2 herbal mixtures containing GC have been analyzed for authentication, adulterations, and/or impurities. In the UK and other European countries, it is not routine practice for the regulatory agency to analyse products in this way. All authorised products have Good Manufacturing Practices (GMP) approval with appropriate batch testing procedures by the manufacturers to control for these issues. Moreover, comedication with synthetic drugs, herbs, herbal mixtures, and dietary supplements was reported for 8 patients.
IndicationThe indication approved by the German regulatory agency BfArM for the herbal drug GC was confined to upper abdominal crampy pains from the biliary tract and the upper gastrointestinal tract,5 various additional indications were listed in published case reports16 and spontaneous reports.17 For all 16 cases of the study group, indications for GC treatment were available (Table 1): they included bloating (cases 1 and 15); atopic eczema (case 2); dyspepsia (case 3); skin complaints (case 4); abdominal pains and discomfort (case 5); biliary spasms (case 6); insomnia (case 7); pyrosis (case 8); upper abdominal pains (cases 9,10,12) with vomiting and gastroesophageal reflux (case 13); bile flow impairment (case 11); general discomfort (case 14); and dyspepsia (case 16). In only 7 patients (cases 5,6,9-13), the indication for treatment was in accordance with the German regulatory approval.
Daily dose, duration of treatment, and cumulative doseAccording to recommendations by the German regulatory agency BfArM, daily doses of 12-30 mg total alkaloids, calculated on the basis of chelidonine as the most active constituent of GC extracts, are considered effective.5 GC tablets or capsules commonly contained 4 mg chelidonine. Only 12 patients reported a daily dose (Table 1): with up to 3 capsules or tablets per day of GC drug in 10 patients, 50 drops of GC extract daily in 1 patient, and 1 cup per day of GC tea in another one. On average, the 10 patients consumed 2.6 capsules or tablets of GC drug per day, corresponding to an average of 10 mg chelidonine. No patient reported evidence for GC overdose when used as GC capsules or tablets.
Maximum treatment duration with GC was not restricted by the BfArM.5 Duration of treatment was assessable in 14 cases and ranged from 3 weeks to 9 months, with 2.4 months on average (Table 1). However, excluding 3 cases with very long intake duration shortened the treatment duration and was from 3 weeks to 4.5 months, with 1.9 months on average. The case of one patient is unusual receiving GC treatment for 9 months (case 1) (Table 1); itching and jaundice appeared after 2 months under GC, but were not recognized as GC related, and GC treatment was continued for another 7 months with stable symptoms.6
For GC capsules or tablets (Table 1), the cumulative dosage was assessable in 7 cases and ranged from 280 to 3,240 mg chelidonine, averaging 839 mg. Omitting case 1 from the analysis because of the unusually long treatment of 9 months, the cumulative dosage in the remaining 6 cases ranged from 280 to 636 mg chelidonine with an average of 438 mg.
Challenge, temporal association, and latency periodThe exact date of start and end of GC use was documented in only 7 cases of the study group, and a general time frame on GC in 9 cases (Table 1). It is important to note that exact dates are not routinely released by regulatory agencies because of data protection concerns to ensure anonymity of individual patients. Both the regulatory agency and the marketing authorisation holder generally have access to exact dates. Overall, temporal association between the development of liver disease and the use of GC was well documented in all 16 cases.
Accurate latency period to first symptoms was assessable in 13 patients (Table 1) and ranged from 3 weeks to 4.5 months, averaging 1.7 months; the total treatment duration was between 3 weeks and 9 months with an average of 2.4 months. Among these 13 cases, in 8 patients the latency period was identical with the length of GC treatment, with GC use ceasing after the appearance of first symptoms. In the remaining 5 patients with evident symptoms, however, treatment was continued over a period ranging from 3 days up to 7 months, averaging 42 days. When excluding the patient with additional GC use for 7 months (Table 1, case 1), in the remaining 4 cases the duration of additional GC treatment after the development of first symptoms ranged from 3 days to 4 weeks with an average of 14 days.
SymptomsIn 15 cases of the study group, symptoms such as weakness, anorexia, nausea, vomiting, abdominal pains, dark urine, pale stool, and itching were reported, though at various degrees with jaundice described in 14 patients as the predominant symptom (Table 1). In some cases, however, symptoms such as abdominal pains and nausea were already preexisting and considered as indication for GC treatment.
Liver enzyme values, hepatobiliary sonography, and liver histologyIn all 16 patients, serum activities of ALT (alanine aminotransferase) and AST (aspartate aminotransferase) were reported as increased (Table 1). ALT values were found in the range of 152 to 4,765 U/L with an average of 1,435 U/L. The associated values of AST were considerably lower and ranged from 89 to 3,235 U/L with an average of 730 U/L. The ratio of ALT: AST ranged from 0.91 to 3.25 and was on average 2.04.
In 14 out of 16 cases, serum activities of ALP (alkaline phosphatase) were increased, while in 1 case ALP was normal, in 1 case not available. In the 14 patients, ALP activities ranged from 249 to 516 U/L with an average of 353 U/L.
In 15 cases, results of hepatobiliary sonography, magnetic resonance tomography (MRT), or endoscopic retrograde cholangiopancreaticography (ERCP) were available, showing no biliary obstruction. In 3 patients, thickening of the gall bladder wall (case 6), hepatomegaly (case 8), and cholecystolithiasis or cholesterol polyps associated with questionable cholecystitis (case 12) were reported as pathological findings (Table 1).
Results of liver histology were available in 12/16 patients with prevailing features of hepatitis, single or confluent liver cell necroses, inflammation, and rarely fibrosis and cholestasis (Table 1).
Dechallenge and reexposureWhereas ALT increases because of GC challenge and liver disease were well documented, ALT normalization corresponding to complete resolution was described in only 6 cases with a time frame of 4 weeks up to 5 months after GC cessation and an average of 3.0 months (Table 1). ALT decrease without normalization after dechallenge has been reported in 9 cases, no information was available in one case (Table 1).
A positive reexposure test with GC was described in 2 patients (cases 1 and 2) (Table 1) and is considered a hallmark for the diagnosis of DILI.24-28 and certainly also of HILI by GC (Tables 2 and 3).
GC hepatotoxicity.
| Typology of GC hepatotoxicity | ||
|---|---|---|
| Classification types | Required individual items for classification of GC hepatotoxicity | Items confirmed for cases and GC |
| • Laboratory classification | ||
| Hepatocellular injury | ALT > 2N with normal ALP, or R ≥ 5 | Yes |
| Cholestatic injury | ALP > 2N with normal ALT, or R ≤ 2 | No |
| Mixed injury | ALT > 2N with increased ALP and 2 < R < 5 | No |
| • Pathogenetic classification | ||
| ° Idiosyncratic form | Unpredictability | Yes |
| Dose independency | Yes | |
| Long and variable latency period | Yes | |
| Low incidence in humans | Yes | |
| Lack of experimental reproducibility | Yes | |
| -Metabolic type | Duration of exposure: 1 week to 12 months | Yes |
| Lack of hypersensitivity features | Yes | |
| Delayed response to reexposure (weeks) | Yes | |
| -Immunologic type | Duration of exposure: 1-5 weeks | No |
| Hypersensitivity features | No | |
| Prompt response to reexposure (1 or 2 doses) | No | |
| ° Intrinsic form | Predictability | No |
| Dose dependency | No | |
| Short and consistent latency period | No | |
| High incidence in humans | No | |
| Experimental reproducibility | No | |
| • Clinical classification | ||
| Acute course | ALT normalization after GC cessation: ≤ 6 months | Yes |
| Chronic course | ALT normalization after GC cessation: > 6 months | No |
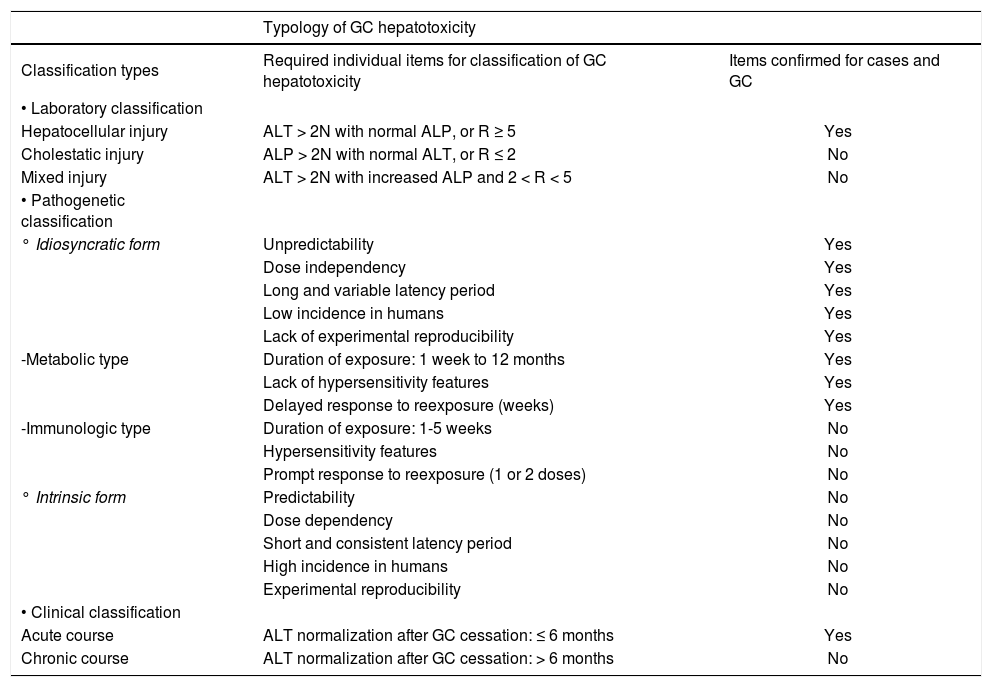
Three different types of classifications may be applied to cases of GC hepatotoxicity with various individual items related to GC and GC hepatotoxicity to be assessed and confirmed. For the laboratory classification of GC hepatotoxicity, values of alanine aminotransferase (ALT) and alkaline phosphatase (ALP) are required as clear criteria for liver injury and for the differentiation between the hepatocellular, cholestatic or mixed hepatocellular-cholestatic pattern.24-26 Laboratory criteria for GC hepatotoxicity are based on values of ALT and/or ALP to be at least 2 N, with N as the upper limit of the normal range. For differentiation between the hepatocellular, cholestatic or mixed hepatocellular-cholestatic pattern of hepatotoxicity, serum ALT and ALP values at the day the diagnosis of GC hepatotoxicity was suspected were evaluated. Each activity is expressed as a multiple of the upper limit of the normal range (N), and the ratio (R) of ALT: ALP is calculated. Hepatocellular liver injury is assumed, if ALT > 2N with normal ALP, or R ≥ 5; cholestatic liver injury is assumed, if there is an increase of ALP > 2N with normal ALT or R ≤ 2; mixed type liver injury is assumed in all other cases, i.e. ALT > 2N, ALP is increased and 2 < R < 5. The pathogenic classification and the clinical classification of GC hepatotoxicity followed previous recommendations for cases of DILI.27 Evidence related to GC and GC hepatotoxicity with the corresponding items is based on the present analysis including data of table 1, and on previous assessments.16,17
Clinical characteristics of GC hepatotoxicity.
| Characteristics: |
|---|
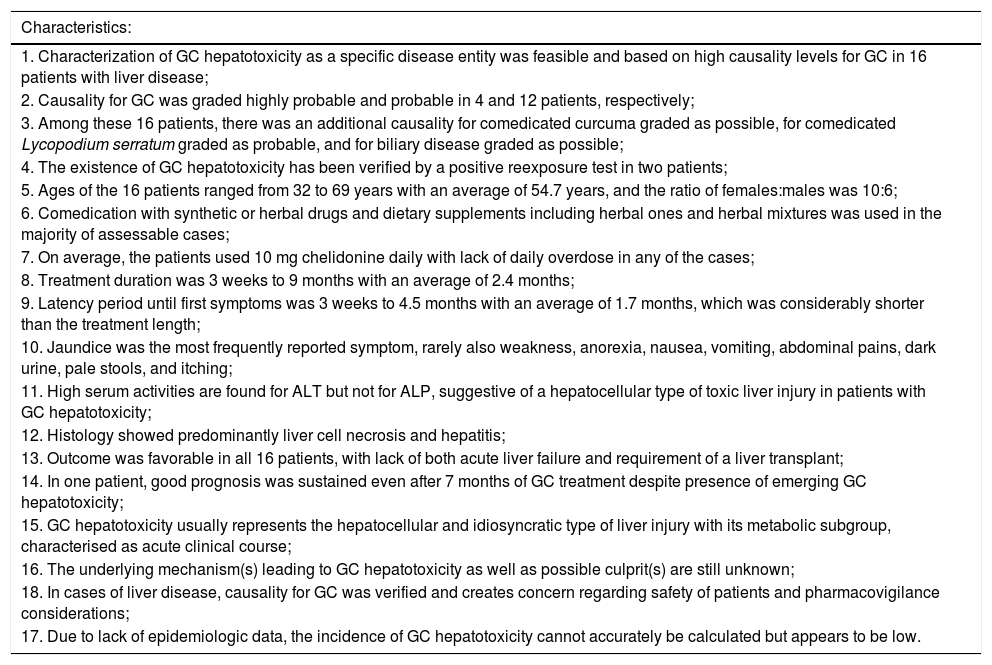
| 1. Characterization of GC hepatotoxicity as a specific disease entity was feasible and based on high causality levels for GC in 16 patients with liver disease; |
| 2. Causality for GC was graded highly probable and probable in 4 and 12 patients, respectively; |
| 3. Among these 16 patients, there was an additional causality for comedicated curcuma graded as possible, for comedicated Lycopodium serratum graded as probable, and for biliary disease graded as possible; |
| 4. The existence of GC hepatotoxicity has been verified by a positive reexposure test in two patients; |
| 5. Ages of the 16 patients ranged from 32 to 69 years with an average of 54.7 years, and the ratio of females:males was 10:6; |
| 6. Comedication with synthetic or herbal drugs and dietary supplements including herbal ones and herbal mixtures was used in the majority of assessable cases; |
| 7. On average, the patients used 10 mg chelidonine daily with lack of daily overdose in any of the cases; |
| 8. Treatment duration was 3 weeks to 9 months with an average of 2.4 months; |
| 9. Latency period until first symptoms was 3 weeks to 4.5 months with an average of 1.7 months, which was considerably shorter than the treatment length; |
| 10. Jaundice was the most frequently reported symptom, rarely also weakness, anorexia, nausea, vomiting, abdominal pains, dark urine, pale stools, and itching; |
| 11. High serum activities are found for ALT but not for ALP, suggestive of a hepatocellular type of toxic liver injury in patients with GC hepatotoxicity; |
| 12. Histology showed predominantly liver cell necrosis and hepatitis; |
| 13. Outcome was favorable in all 16 patients, with lack of both acute liver failure and requirement of a liver transplant; |
| 14. In one patient, good prognosis was sustained even after 7 months of GC treatment despite presence of emerging GC hepatotoxicity; |
| 15. GC hepatotoxicity usually represents the hepatocellular and idiosyncratic type of liver injury with its metabolic subgroup, characterised as acute clinical course; |
| 16. The underlying mechanism(s) leading to GC hepatotoxicity as well as possible culprit(s) are still unknown; |
| 18. In cases of liver disease, causality for GC was verified and creates concern regarding safety of patients and pharmacovigilance considerations; |
| 17. Due to lack of epidemiologic data, the incidence of GC hepatotoxicity cannot accurately be calculated but appears to be low. |
Although jaundice was present in virtually all patients of the study group, clinical outcome was favorable after discontinuation of GC treatment. No case progressed to acute liver failure or required a liver transplant. Good outcome was also described in 1 patient (case 1) (Table 1) who continued GC treatment for another 7 months although itching and jaundice appeared already 2 months after initiation of GC therapy.6 When GC use was stopped after 9 months, there was a spontaneous regression of the aminotransferases.
Liver Injury by GC and ClassificationPrinciples of disease classification have primarily been established for cases of DILI,26-28 but were subsequently also applied to cases of HILI.16-18,22,23,29-31 There are three different approaches to the classification (Table 2); the first is based on laboratory values of ALT and ALP,24-26 the second on pathogenetic mechanisms of toxic liver injury,27,28 and the third on the clinical course.27 Some of these classifications have been applied to published case reports16 and spontaneous reports17 of GC hepatotoxicity: details of all cases of the study group will be considered according to these classification principles (Table 2).
Laboratory classificationThe laboratory classification of GC hepatotoxicity (Table 2) requires initial data of ALT and ALP values (Table 1) to differentiate between hepatocellular, cholestatic, or the mixed type injury that represents the combination of cholestatic and hepatocellular type (Table 2). Based on the initial ALT and ALP values (Table 1), there is clear evidence that GC hepatotoxicity represents the hepatocellular type of injury in 14 out of 16 cases, with R values of 12.9 up to 83.3 (Tables 2 and 2). In the remaining 2 cases, R was 3.0 in one patient (case 3) (Table 1) in line with a mixed hepatocellular-cholestatic pattern (Table 2), R was not assessable in the other patient (case 8) due to lack of ALP data (Table 1). No case indicated a cholestatic pattern of injury.
Pathogenetic classificationThe pathogenetic classification of GC hepatotoxicity requires a variety of items to differentiate between the idiosyncratic form, which is unpredictable and dose independent, and the intrinsic form that is predictable and dose dependent (Table 2). In cases of GC hepatotoxicity, data typical for the intrinsic form are lacking (Tables 1 and 2), especially predictability;16,17 dose dependency and/or daily overdose;5,16,17 short and consistent latency period (Table 1); high incidence among GC users;5 and reproducibility in animal experiments.32 Prevailing features of GC hepatotoxicity therefore are not compatible with the intrinsic type of injury but are rather strongly suggestive for the idiosyncratic variety. Indeed, GC hepatotoxicity is characterized by typical items of idiosyncracy (Tables 1 and 2) like unpredictability,5 dose independency including lack of daily overdose, based on approved GC doses from 8-12 mg chelidonine/d contained in 2-3 tablets or capsules/d (Table 1); long and variable latency periods (Table 1); low incidence in GC users;5 and lack of reproducibility in experimental animals.32
In order to further subclassify idiosyncratic GC hepatotoxicity, the metabolic and the immunologic subtype have to be separated (Table 2).27 The immunologic subtype appears unlikely to apply for GC hepatotoxicity since conditions such as short duration of exposure of 1-5 weeks, features of overt hypersensitivity, and prompt response to reexposure with 1-2 doses are lacking (Tables 1 and 2). However, the cases exhibit various characteristics suggestive of the metabolic subtype of hepatotoxicity.16,17 Among these items are a variable duration of exposure of one week up to 12 months, the absence of clinical features of hypersensitivity such as rash, fever, and eosinophilia, and the delayed response to rechallenge of many days or weeks,27 as shown in the analysis of case data16,17 (Tables 1 and 2). A weak dose dependency in susceptible humans who adhere to recommended doses, another facultative criterion of the metabolic subtype,27 may be derived from the assessed cases (Table 1). Overall, GC hepatotoxicity is best described as the metabolic subtype of an idiosyncratic reaction, based on a rare metabolic aberration in susceptible humans.
Clinical classificationThe clinical classification of GC hepatotoxicity indicates an acute rather than a chronic course in all cases (Table 2), since normalization of ALT occurred between 4 weeks and 5 months and therefore below the limit of 6 months (Table 1).
Overall classificationWhen the laboratory, pathogenetic, and clinical classifications are combined, GC hepatotoxicity emerges as a specific form of a hepatocellular pattern of injury, based on an idiosyncratic reaction with a metabolic subtype caused by a metabolic aberration, and with features of a clinically acute liver disease.
GC Hepatotoxicity as a Typical HiliGC hepatotoxicity as a typical HILI reaction (Table 3) may well be characterized on a positive reexposure test in 2 cases, associated with a highly probable causality for GC in 4 patients and a probable causality in 12 cases (Table 1), as well as on additional features of its classification (Table 2). In these 16 cases with GC hepatotoxicity, female patients prevail (Table 3), in line with other cases of HILI29-31 and DILI.27 Jaundice was present in virtually all cases, combined with high values of ALT and AST up to over 4,000 U/L (Table 1), which normally signifies a severe clinical course and poor prognosis.33 Surprisingly, outcome in all 16 patients with GC hepatotoxicity was favorable (Tables 1 and 3). In addition, outcome was still good even when GC treatment was continued for another 7 months after jaundice appeared 2 months after start of GC use.6 This course is quite unusual but should be a reminder for clinicians to thoroughly evaluate also the herbal product use in any patient with overt liver disease including jaundice of undetermined cause of etiology.
GC hepatotoxicity is likely due to idiosyncrasy (Table 2) by a metabolic aberration in susceptible individuals.27 Any of the ingredients of GC extracts may fulfil criteria as a culprit for human GC hepatotoxicity. Among the more than 20 known ingredients are various biologically active isoquinoline alkaloids including chelerythrine, chelidonine, isochelidonine, sanguinarine, berberine, coptisine, dihydrocoptisine, stylopine, and protopine.5,34 These compounds lack experimental hepatotoxicity in vivo in animals32,35 with GC application at doses 50 and 100 times higher than those generally used in humans,32 in support of the idiosyncratic nature of GC hepatotoxicity (Table 2). Cytotoxicity studies in vitro with hepatocytes of different species using GC extracts or their individual ingredients provided variable results at high concentrations;5,35,36 their results cannot prove or disprove an idiosyncratic reaction. At present, therefore, evidence for a single culprit in GC extracts is lacking.
Concluding RemarksGC hepatotoxicity is a typical HILI with a sound causality track for GC as culprit but the specific causal ingredient has not yet been defined. Causality levels for GC have been established in 16 cases as highly probable and probable, indicating high causality gradings. Based on laboratory, pathogenetic, and clinical assessment, GC hepatotoxicity is best characterized as the hepatocellular type of injury, based on an idiosyncratic reaction with its metabolic subtype, and associated with features of an acute clinical course. The description of thorough investigations associated with the evaluation of the GC cases may have impact on future evaluations of HILI cases by other herbal drugs and herbal supplements.
Conflict of InterestThe authors declare that they have no conflict on interest.