Aim. To determine the incidence and factors associated with drug induced hepatic dysfunction in children on anti-tuberculous treatment (ATT).
Setting. Pediatric Tuberculosis Clinic at B.J. Wadia Children’s Hospital, Mumbai.
Material and methods. 46 children with tuberculosis on ATT between April 2007 and February 2008 were included. Serum glutamic pyruvic transaminase (SGPT) level was measured at the beginning, after 15 days of starting ATT, at the end intensive phase and then if the patient developed symptoms of hepatic dysfunction. A value 3 times the normal value of the testing laboratory was considered to be significant for liver dysfunction. Liver dysfunction was analysed for association with factors like age, sex, weight, malnutrition, type of tuberculosis and severity of tuberculosis using SPSS Statistics software, Ver-sion15.0.
Results. Seven (15.2 %) out of 46 children developed drug induced hepatic dysfunction, of which 2 (28.6%) patients had 2 episodes of liver dysfunction while 5 (71.4%) had 1 episode of liver dysfunction. One (14.3%) developed symptom of hepatitis in the form of jaundice and hepatomegaly. All the patients developing liver dysfunction were in the intensive phase of treatment. The mean age of the children developing liver dysfunction was 4.0 ± 3.76 years. Liver dysfunction was associated with age younger than 3/ years (p = 0.025). Liver dysfunction was not associated with sex, weight, malnutrition, type of tuberculosis and severity of tuberculosis.
Conclusion. Regular monitoring of SGPT levels is recommended in all children on ATT below the age of 3 / years.
Tuberculosis is one of the foremost health problems in India. The single biggest problem in the treatment of TB is drug induced liver dysfunction, which has a mortality of up to 13%.1,2 Pyrazinamide (PZA), Isoniazid (INH) and Rifampicin (RIF) are hepatotoxic drugs in decreasing toxicity.3 The mortality in children whose SGPT levels rise to < 3 times normal and if Isoniazid is continued, is as much as 50%.4 Drug induced liver dysfunction also contributes to the development of multi drug resistance as it entails stoppage of treatment. Many factors have been implicated in the development of drug induced liver dysfunction.5,6 However data on incidence and factors involved in liver dysfunction in children in India is scarce. Therefore this study was undertaken to determine the incidence and the factors associated with liver dysfunction.
Material and MethodsThis is a retrospective study undertaken at a tertiary pediatric hospital, B.J. Wadia hospital. Fortysix children coming to the clinic between 27 th April 2007 and 22nd February 2008 and diagnosed to have tuberculosis were included in the study. Diagnosis of tuberculosis was based on microscopy, culture, Mantoux test, contact with TB and radiological findings. The children were started on anti-tuberculosis treatment. They were given a combination of INH 5 mg/kg/day, RIF 10 mg/kg/day, PZA 25 mg/kg/day and Ethambutol (E) 15 mg/kg/day1 in a schedule as per 1997 Indian Academy of Pediatrics (IAP) consensus statement.7 The liver function test, serum glutamic pyruvic transaminase (SGPT) level was measured at the baseline, after 15 days of starting anti-tuberculosis treatment, at the end intensive phase and then if the patient developed symptoms of liver dysfunction such as hepatomegaly or jaundice. SGPT more than 3 times the normal value of the baseline was considered to be significant for liver dysfunction.8 The children were classified as malnourished if they were below the 3rd percentile for their growth as per age according to the Agrawal Growth charts.9
Incidence of hepatic dysfunction was determined and factors such as age, sex, weight, malnutrition, type of tuberculosis (pulmonary or extra-pulmonary) were compared for any association with liver dysfunction using chi-square test for proportions and analysis of variance (ANOVA-1 way) for continuous data. The software used was SPSS Statistics, Version 15.0. A P < 0.05 was considered as significant.
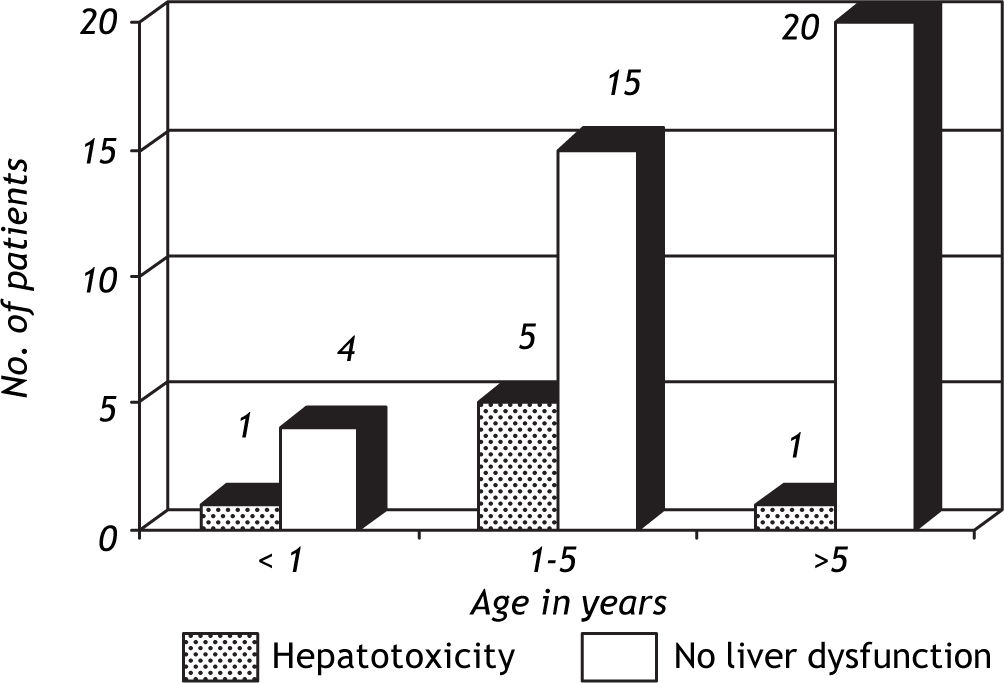
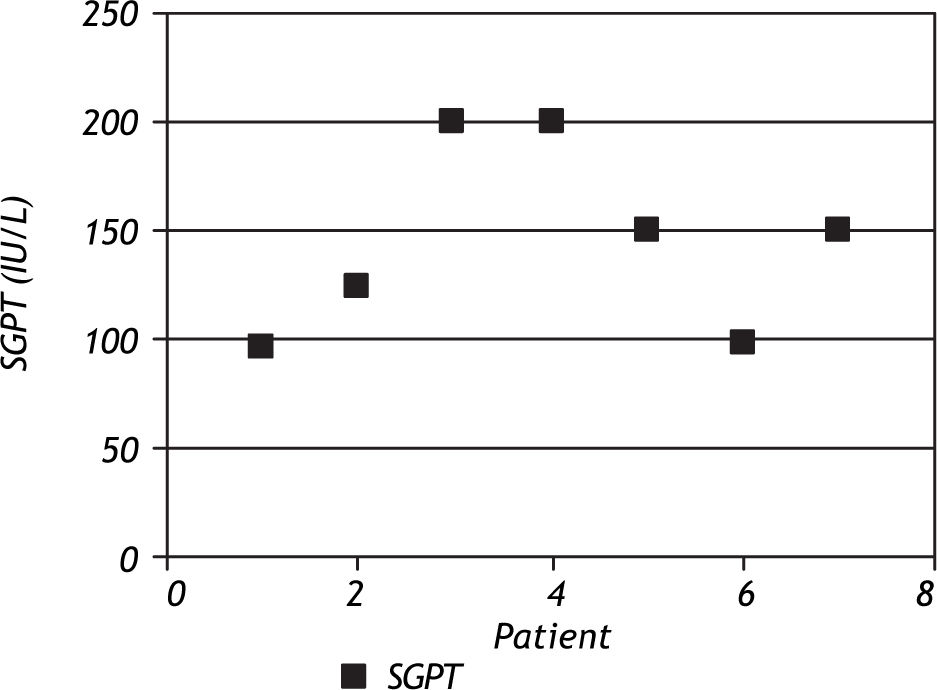
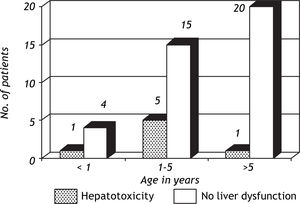
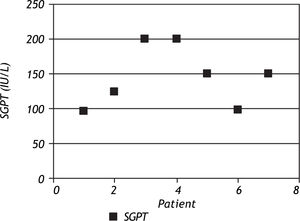
ResultsOut of the 46 children on anti-tuberculosis treatment, 7 developed liver dysfunction (15.2%), of which 2 (28.6%) patients had 2 episodes of liver dysfunction while 5 (71.4%) had 1 episode of liver dysfunction. All the patients developing liver dysfunction were in the intensive phase of treatment. Patients included in the study ranged between 4 months and 13.5 years, and the mean age was 5.88 ± 3.74 years. Age groups of patients with and without hepatotoxicity are depicted in figure 1. Patients who developed hepatotoxicity had a mean age of 4.0 years, whereas the mean age among the patients not developing hepatotoxicity was 6.2 years. Four (36.4%) out of the 11 patients below the age of 3 % years developed liver dysfunction, whereas 3 (9.375%) out of the 35 patients above 3% years developed liver dysfunction, which was statistically significant (p = 0.025). Most of the patients had asymptomatic elevation of SGPT (Figure 2); however, 1(14.3%) patient presented with hepatomegaly and jaundice. Male:female ratio was 1.3:1. However, 20% girls developed liver dysfunction whereas 11% boys developed liver dysfunction which was not statistically significant (p = 0.627).
Patients who developed hepatotoxicity had a mean weight of 16.1 kg ± 8.6, whereas the mean weight among the patients not developing hepatotoxicity was 17.8 kg ± 9.1 which was not statistically significant (p = 0.647). Malnutrition showed no association with liver dysfunction. Only 1 (14.3%) of the children developing liver dysfunction was malnourished (p = 0.517).
Out of the 7 patients developing liver dysfunction, only 1(14.3%) had pulmonary TB while 6 (85.7%) had extra-pulmonary TB, but was not statistically significant (p = 0.372).
DiscussionIncidence of drug induced hepatitis ranging from 2.6 to 41% in children has been reported previously.5,6,10,11 Incidence among Indian children reported earlier is 2.6%11 though higher incidences have been reported in Japan (8.08%)5 and Africa (29.2%).6 The mortality in children whose SGPT levels rise to > 3 times normal and if Isoniazid is continued, is as much as 50%.4 In our study 15% had hepatic enzyme derangement and all in intensive phase where the patients are on either 3 drugs or 4 drugs Anti tuberculous therapy (ATT). This may be due to regular testing for liver enzymes that was done in our patients during intensive phase of treatment and picking up patients who were asymptomatic as out of the 7 patients, only one patient has symptomatic liver dysfunction which is similar to the study by Okhawa, et al.5 The studies in which the incidence is found to be higher, used a higher dose of isoniazid and rifampicin (15-20 mg/kg of isoniazid and 15 mg/kg of rifampicin)12 whereas in our patients the doses of INH were 5 mg/kg/day and rifampicin was 10 mg/kg/day. However all our patients were on combined INH, rifampicin and PZA at the time they had liver dysfunction.
Isoniazid, rifampicin and pyrazinamide are hepatotoxic drugs. Isoniazid causes hepatitis due to the formation of hydrazines. They are formed by the action of P450 on acetylhydrazine, a product of isoniazid metabolism in the liver. The hydrazines get covalently bound to liver proteins thus damaging the cells. Higher plasma levels of isoniazid do not increase the risk of hepatitis.13 Rifampicin induces P450 enzymes and therefore increases the risk of hepatotoxicity when given with isoniazid. Hepatitis due to rifampicin alone occurs in 1% or less of patients.14 Pyrazinamide on the other hand causes direct liver toxicity which is dose related. It is hydrolyzed by a microsomal deaminase to the active metabolite, pyrazinoic acid, which is then hydroxylated by xanthine oxidase to 5-hydroxypyrazoinoic acid. These are eliminated renally.14
The younger age group of children is more prone to developing liver dysfunction. In our study we found that liver dysfunction was significantly more common in children below 3% years of age. Another study in Japan reported an association with age less than 5 years.5 Therefore periodic liver function test monitoring would be recommended in this age group.
Factors associated with drug induced hepatitis as found by studies done on adults are old age,15 female sex,16,17 lower pretreatment serum albumin,15,17 moderately or far advance disease,15,16 high alcohol in-take,15,16 slow acetylators of N-acetyltransferase 2,18 low body mass index,19 malnutrition,17 HIV and HCV coinfection20 and decreased glutathione-S-transferase activity.18 We found that sex, weight, malnutrition and type of tuberculosis were not related to development of liver dysfunction as also found by other studies in children.5,10 However malnutrition has been found to be a risk factor by some studies.6,21
Mortality rates of ATT drug induced hepatitis depend critically on early detection. If drug therapy is discontinued promptly when a 3-fold or greater transaminase elevation occurs, mortality should be negligible. In contrast, if isoniazid is continued after this point or after symptoms develop, mortality due to hepatic failure may exceed 50% unless liver transplantation is performed.4 Thus diligent monitoring would be essential.
Thus to conclude, liver dysfunction in children on ATT is common especially in children less than 3% years of age and those on combined INH, rifampicin and PZA in intensive phase. Therefore we recommend regular monitoring of SGPT levels in children with TB on treatment, especially during the intensive phase. The 1997 Indian Academy of Pediatrics (IAP) consensus statement7 and the latest WHO guidelines 2006 for children22 state that biochemical monitoring is not required. A revision of the guidelines maybe required, monitoring being made essential.