Background. Renal failure (RF) is reported to occur in 11-49% of the patients with decompensated end-stage liver disease (ESLD) and has been associated with increased mortality, particularly in the occurrence of hepatorenal syndrome (HRS) type 1. Aims. To evaluate the frequency and outcome of RF in patients admitted to the hospital due to decompensated ESLD and to assess the impact of the underlying cause of RF on survival.
Material and methods. Four hundred and six patients (65% males, mean age 62 ± 12 years) with decompensated ESLD were evaluated for the occurrence of RF (defined as serum creatinine 3 1.5 mg/mL). The underlying cause of RF was reckoned in each subject and compared to outcome.
Results. Renal failure was observed in 39% of the patients at admission and in 10% of the subjects during hospitalization. Mortality was significantly higher in subjects with RF (26 vs. 1%, p < 0.000001). Hypovolemia, bacterial infections, parenchymal kidney diseases and HRS were identified as causes of RF in, respectively, 40, 32, 15 and 12% of the cases. Mortality was significantly higher in those subjects with HRS type 1 and bacterial infections, when compared to other causes of RF.
Conclusions. Renal failure occurs in nearly half of the patients with decompensated ESLD. It is most commonly caused by hypovolemia and bacterial infections. Occurrence of RF has an adverse impact in patient survival, particularly in those subjects with bacterial infections and HRS type 1, prone to develop progressive renal dysfunction despite intensive medical care.
Renal failure (RF) is reported to occur in 11% of cirrhotic patients admitted to the hospital due to upper gastrointestinal bleeding (UGB),1 in 27-34% of the patients with infections2-4 and in 40-49% of critically-ill subjects with cirrhosis admitted to the intensive care unit (ICU).5,6 In those subjects, RF was associated either with increased intra-hospital mortality, as well as shortened survival after hospital discharge.5,6 Likewise, the occurrence of RF in outpatients with end-stage liver disease (ESLD) has been also associated with decreased survival7,8 and creatinine levels has been incorporated in the Model for End-Stage Liver Disease (MELD), designed to predict severity of ESLD and mortality in the liver transplantation waiting list.9
The pathogenesis of RF in cirrhosis is multifactorial.8,10 In this regard, distinct causes, not mutually exclusive, were associated with renal impairment in patients with ESLD, including bacterial infections, hepatorenal syndrome (HRS) types 1 and 2, hypovolemia, parenchymal kidney diseases, and nephrotoxicity due to drugs or contrast agents.8,10 Hepatorenal syndrome type 1 is the result of intense renal vasoconstriction due to worsening of the circulatory dysfunction due to ESLD and is associated with a poor prognosis,10,11 particularly in non-responders to standard therapy with albumin and splancnic vasoconstrictors.10,11 Bacterial infections, either spontaneous bacterial peritonitis,2,3,12 as well as other infections4,12 can also alter the splancnic and systemic circulation of cirrhotics and lead to RF that may regress after control of the infection episode or evolve to full-blown HRS type 1.3,4,12
Recently, the prognosis of RF in subjects with ESLD was shown to vary depending on the cause of renal impairment and severity of liver disease.13 In this respect, RF due to parenchymal kidney diseases and hypovolemia were reported to have a much better prognosis, when compared to RF attributable to bacterial infections or HRS type 1.13
The purpose of the present study was to investigate the prevalence of RF in patients with cirrhosis admitted to the hospital due to decompensated ESLD, as well as to reassess the impact of the cause of RF in patient’s survival.
Material and MethodsPatients with decompensated ESLD consecutively admitted to the Unit of Gastroenterology and Hepatology of the Portuguese Hospital of Salvador, Bahia, Brazil from January 2005 to December 2007 were retrospectively evaluated, employing a prospectively collected database. This unit is focused on the treatment of liver diseases and on the postoperative care of liver transplant patients and is a referral center for patients with ESLD. Decompensation of ESLD was defined by the occurrence of variceal bleeding, hepatic encephalopathy, infections and tense ascitis as well as any other acute clinical event requiring hospitalization. The diagnosis of ESLD was based on clinical, biochemical and echographic findings, as well as on liver histology, whenever a liver biopsy specimen was available. The etiology of ESLD and the reason for hospitalization was established in all patients. In the case of more than one cause for admission, the main cause was reckoned based on the following hierarchy: upper digestive bleeding, infection, hepatic encephalopathy, tense ascitis and others.14 Clinical and biochemical parameters associated with ESLD were sought in each patient. Severity of ESLD was assessed by the Child-Pugh score (CPS) and the MELD score.9,15
Renal failure was arbitrarily defined as the presence of creatinine levels equal or higher than 1.5 mg/mL either at admission or during hospitalization.16 Renal failure was classified in distinct groups including: bacterial infections, hypovolemia, parenchymal or parenchymal kidney diseases, HRS and contrast or drug-induced RF.8,10,13 Hypovolemia was assumed as a cause for renal impairment in the presence of gastrointestinal hemorrhage or dehydration ascribed to gastrointestinal fluid losses or diuretic usage as well as in the occurrence of RF remission with the administration of intravenous saline. Prior diagnosis of parenchymal kidney disease, past history of use of drugs or contrast agents was elicited in each patient as part of a protocol. Bacterial infections were sought in every hospitalized patient by urine analysis and culture and blood cultures. Ascitic fluid analysis and culture were performed in every patient with ascitis, whereas chest X rays were obtained whenever there was a clinical suspicion of respiratory tract infection. Renal failure was attributed to infection whenever the infection episode was suspected or documented within 48 h of the elevation of creatinine levels. Those subjects with presumed infections were initially treated with empiric antibiotics based on the most probable site of infection. Tailoring of antimicrobial therapy was subsequently based upon laboratory and radiology data as well as culture and sensitivity results.
The diagnosis of HRS types 1 and 2 were defined according to the non-revised criteria of the International Ascitis Group.16 Briefly, HRS-1 was suspected in all subjects based on the presence of creatinine levels greater than 1.4 mg/mL either at admission or during hospitalization doubling to levels higher than 2.5 mg/mL within two weeks in the absence of shock, hypovolemia, bacterial infections or known exposure to nephrotoxic agents or a recognizable etiology for chronic renal failure. Failure to attain creatinine levels under 1.4 mg/mL 24 h after volume expansion with at least 1,500 mL of cristalloids or 72 h after institution of antibiotics with apparent control of infection were indicative of the presence of HRS, that was confirmed after exclusion of possible causes of intrinsic renal diseases by 24 h urine protein excretion levels lower than 500 mg/day, as well as by the absence of renal parenchyma abnormalities at the ultrasound. Therapy with terlipressin and high-dose albumin was considered for all patients with HRS-1. Outcomes for those patients were previously described elsewhere.11
In the case of more than one cause for renal failure, the main cause was reckoned based on the following hierarchy: HRS, bacterial infections, drug-induced renal failure and hypovolemia. Subjects with end-stage renal failure were considered in the group of parenchymal kidney diseases even if they had acute-on chronic RF induced by the other aforementioned etiologies. All patients were followed until hospital discharge or death. Outcomes were assigned as reversal, stabilization or progression of RF based on comparison of creatinine levels at the diagnosis of renal failure and at hospital discharge. Reversal was assumed in the occurrence of normalization of creatinine levels at discharge. In the absence of reversal, three distinct intervals of creatinine levels were created for comparisons:
- •
Under 2 mg/mL.
- •
Between 2-3 mg/dL.
- •
Above 3 mg/mL.
Stabilization was considered whenever the creatinine level at discharge remain below or in the same interval of the creatinine level at the diagnosis of RF in the absence of reversal, whereas progression was considered whenever the creatinine levels at discharge remains above the interval of the creatinine at diagnosis of renal impairment.
The study was approved by the Ethics Committee in Research of the Portuguese Hospital of Salvador, Bahia.
Statistical analysisThe differences between groups of patients were compared using either the Mann-Whitney test or the Fisher exact probability test, when appropriate. A p value < 0.05 was considered significant. Clinical data are presented in text and tables as mean and standard deviation.
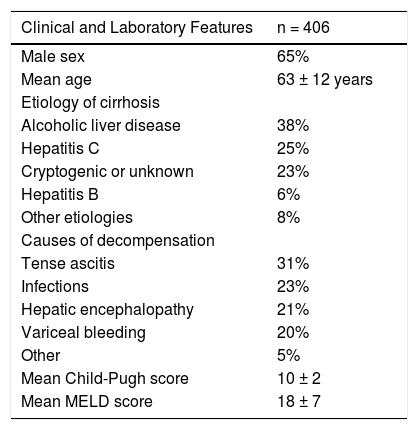
ResultsFour hundred and six patients (65% males, mean age 62 ± 12 years) with the diagnosis of decompensated ESLD were evaluated. Clinical and laboratory parameters of those subjects are described in table 1. Most of the subjects had ESLD due to either alcoholic liver disease or hepatitis C with a mean CPS and MELD score of 10 ± 2 and 18 ± 7, respectively.
Clinical and laboratory features of patients with decompensated end-stage liver disease.
| Clinical and Laboratory Features | n = 406 |
|---|---|
| Male sex | 65% |
| Mean age | 63 ± 12 years |
| Etiology of cirrhosis | |
| Alcoholic liver disease | 38% |
| Hepatitis C | 25% |
| Cryptogenic or unknown | 23% |
| Hepatitis B | 6% |
| Other etiologies | 8% |
| Causes of decompensation | |
| Tense ascitis | 31% |
| Infections | 23% |
| Hepatic encephalopathy | 21% |
| Variceal bleeding | 20% |
| Other | 5% |
| Mean Child-Pugh score | 10 ± 2 |
| Mean MELD score | 18 ± 7 |
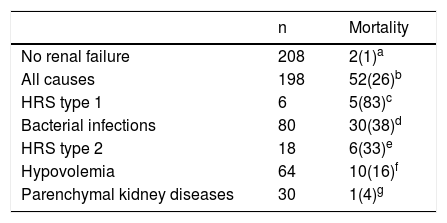
Based on the aforementioned criteria, 159 (39%) patients had RF at admission and 39 (10%) developed RF during the course of hospitalization. The main causes of RF are depicted on table 2. Bacterial infections and hypovolemia were responsible for most of the cases, whereas HRS was seen in only 12% of the patients with RF.
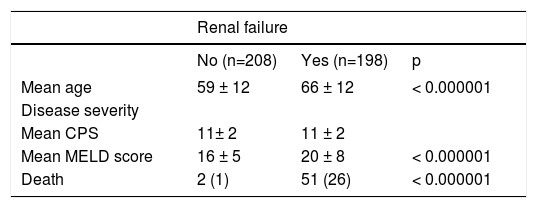
Patients with RF either at admission or during the course of hospitalization were older (66 ± 12 years vs. 59 ± 12 years, p < 0.000001) and, as expected, had higher MELD scores (20 ± 8 vs. 16 ± 5, p < 0.000001), when compared to their counterparts without RF (Table 3). In-hospital mortality was observed in 53 (13%) patients and was higher in subjects with RF when compared to patients without RF (26 vs. 1%, p < 0.000001), particularly in respect to RF due to HRS type 1 and bacterial infections (Table 4). Overall, patients with RF, with exception of those with parenchymal kidney disease, had higher mortality, when compared to their counterparts without RF. In regard to the cause of RF, mortality in the group of patients with HRS type 1 was significantly higher, when compared to other groups with the exception of HRS type 2 (Table 4). On the other hand, subjects with RF due to hypovolemia and parenchymal kidney diseases had lower mortality, when compared to their counterparts with HRS type 1 and with bacterial infections. In addition, subjects with parenchymal kidney diseases have lower mortality even when compared to their counterparts with HRS type 2 (Table 4).
Clinical and laboratory features of cirrhotic patients according to the presence of RF.
| Renal failure | |||
|---|---|---|---|
| No (n=208) | Yes (n=198) | p | |
| Mean age | 59 ± 12 | 66 ± 12 | < 0.000001 |
| Disease severity | |||
| Mean CPS | 11± 2 | 11 ± 2 | |
| Mean MELD score | 16 ± 5 | 20 ± 8 | < 0.000001 |
| Death | 2 (1) | 51 (26) | < 0.000001 |
Numbers in parentheses are percentages.
Mortality of cirrhotic patients in the ICU according to the occurrence and underlying cause of RF.
| n | Mortality | |
|---|---|---|
| No renal failure | 208 | 2(1)a |
| All causes | 198 | 52(26)b |
| HRS type 1 | 6 | 5(83)c |
| Bacterial infections | 80 | 30(38)d |
| HRS type 2 | 18 | 6(33)e |
| Hypovolemia | 64 | 10(16)f |
| Parenchymal kidney diseases | 30 | 1(4)g |
Numbers in parentheses are percentages. abp < 0.000001. acp = 0.0001. adp < 0.0000001. aep = 0.000003. afp = 0.00001. agp = NS. cdp = 0.04. cep = NS. cfp = 0.001. cgp = 0.00009. dep = NS. dfp = 0.005. dgp = 0. 0001. egp = 0.008. efp =NS. gfp=NS.
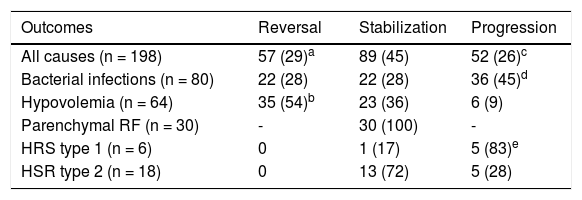
Reversal of RF, as previously defined, was seen in 57 (29%) of the affected subjects, whereas stabilization and progression was observed in, respectively, 89 (45%) and 52 (26%) of the cases. The outcome of RF was shown to vary markedly according to the cause of renal impairment. When compared to all other causes of RF, reversal was seen more often in subjects with hypovolemia (54 vs. 29%, p = 0.0003), whereas progression was more frequently associated with HRS type 1 (83 vs. 26%, p = 0.007) and bacterial infections (45 vs. 26%, p = 0.004) (Table 5).
Outcomes of RF observed at admission or during hospitalization in cirrhotic patients according to the cause of renal impairment.
| Outcomes | Reversal | Stabilization | Progression |
|---|---|---|---|
| All causes (n = 198) | 57 (29)a | 89 (45) | 52 (26)c |
| Bacterial infections (n = 80) | 22 (28) | 22 (28) | 36 (45)d |
| Hypovolemia (n = 64) | 35 (54)b | 23 (36) | 6 (9) |
| Parenchymal RF (n = 30) | - | 30 (100) | - |
| HRS type 1 (n = 6) | 0 | 1 (17) | 5 (83)e |
| HSR type 2 (n = 18) | 0 | 13 (72) | 5 (28) |
Numbers in parentheses are percentages. abp = 0.0003. cdp = 0.004. cep = 0.007.
In the present investigation, RF was observed in 39% of the patients admitted to the hospital due to decompensated ESLD and in 10% of those subjects during the course of hospitalization. It is important to highlight that our study was retrospective, but our data is quite similar to other reports which described RF in 11-34% of patients hospitalized due to ESLD based on abnormalities in serum creatinine levels.1-4,12 Higher frequencies of RF were reported by other investigators when cirrhotic subjects admitted to the ICU were evaluated using the RIFLE system, which has a better performance for establishing RF when compared to serum creatinine levels, because it takes into account changes in creatinine levels, as well as in urinary output.17 In this regard, RF was disclosed in 40-49% of those critically ill subjects with ESLD using this scoring system.5,6
MELD score and age were recognized as risk factors for RF in the present investigation. Higher MELD scores were invariably associated with the occurrence of RF in several studies,12,18,19 but older age was only associated with RF in one prospective study that assessed the frequency of RF in outpatients with ESLD.20
The causes of RF encountered in the current study were:
- •
Bacterial infections (40%).
- •
Hypovolemia (32%).
- •
Parenchymal kidney disease (15%).
- •
HRS type 2 (9%) and type 1 (3%).
Other studies have reported variable frequencies of the aforementioned etiologies for RF in hospitalized subjects with cirrhosis,13,18 as well as in outpatients.20 Martin-Llahi, et al.13 have investigated the causes of RF in hospitalized patients with ESLD and have found quite similar results with infections, hypovolemia, HRS and parenchymal kidney disease accounting, respectively, for 46, 32, 13 and 9% of the cases. Lower frequencies of RF were reported by Montoliu, et al.20 analyzing a different type of cohort of cirrhotic outpatients, who were prospectively followed for 41 ± 3 months, after the onset of ascitis. The authors have found RF due to hypovolemia, infections and HRS in 27, 14 and 7.6% of those subjects, respectively. When compared to other studies, only Schepke et al.18 have found HRS type 1 as the main cause of RF in hospitalized patients with ESLD. This heterogeneity is due mainly to selection bias, but also due to the different criteria employed to ascertain the cause of RF. In the present study we have used the non-revised criteria of the International Ascitis Club for the diagnosis of HRS type 116 and it is clear that a great proportion of subjects with RF due to infections would be reclassified as patients with HRS type 1, based on the current employed criteria.21
Mortality was significantly associated with the occurrence of RF. In this respect, 26% of those subjects with RF vs. 1% of their counterparts without RF died in the current investigation. Several reports have disclosed reduced long-term survival of patients with ESLD after the onset of RF.7,8,20 In hospitalized subjects with decompensated ESLD, mortality was associated with the occurrence of RF,1,7,12,18 as well as with its severity, when assessed by the RIFLE score.5,6,22 In patients admitted to the ICU, the prognosis is even more dismal, with reported mortality rates of 65-81%,5,6,23-25 rising to more than 90% in subjects requiring either dialysis and/or inotropic support.26 It has to be pointed out, however, that RF in those subjects is part of the multiple organ dysfunction syndrome due to either septic shock and/or liver failure which carry an ominous prognosis in patients with ESLD.27,28
In the present investigation, even though retrospective, mortality ascribed to RF was shown to vary according to its cause. In this respect, hypovolemia and renal parenchymal diseases were associated with lower mortality when compared to bacterial infections and HRS. Moreover, RF due to hypovolemia and bacterial infections were shown to regress in approximately one third of the patients. These results were also observed in other studies, where higher mortality was restricted to those subjects with HRS-1 and bacterial infections.13,20 Hepatorenal syndrome type 1 was shown to carry a dismal prognosis and has been associated with a high risk of mortality that was independent of the MELD score.18,19 Bacterial infections, particularly when severe or uncontrolled, were also associated with progression either to HRS and/or acute tubular necrosis due to septic shock and high mortality rates.3,4,12
In summary, renal failure occurs in nearly half of the patients admitted to the hospital with decompensated ESLD, particularly in older subjects and in patients with more advanced liver disease assessed by the MELD score. Hepatorenal syndrome type 1 is infrequently seen and most of the cases of RF are due to bacterial infections and hypovolemia. The occurrence of RF had an adverse impact in patient survival, particularly in those subjects with bacterial infections and HRS type 1, prone to develop progressive renal dysfunction despite intensive medical care. These findings, altogether, have to be taken into consideration when assessing prognosis in subjects with cirrhosis and RF.
Abbreviations- •
RF: renal failure.
- •
ESLD: end-stage liver disease.
- •
HRS: hepatorenal syndrome.
- •
UGH: upper gastrointestinal bleeding.
- •
ICU: intensive care unit.
- •
MELD: model for end-stage liver disease.
- •
CPS: Child-Pugh score.