Pleural effusions develop in 6-10% of patients with endstage liver disease. Although, commonly seen in conjunction with ascites, isolated hepatic hydrothorax can occur in a small number of patients with cirrhosis. Refractory hepatic hydrothorax particularly poses a challenging therapeutic dilemma as treatment options are limited at best in these patients. Current pathophysiologic understanding of this disorder, as a cause, points towards the presence of diaphragmatic defects responsible for the shift of fluid from the peritoneal to the pleural cavity. When sodium restriction and diuretic treatment fail, liver transplantation remains the most definitive therapy in these refractory cases. However, transjugular intrahepatic porto-systemic shunt (TIPS), or video-assisted thoracoscopic (VATS) repair of the diaphragmatic defects (with or without pleurodesis) are effective strategies in those who are not transplant candidates or those awaiting organ availability. Hepatic hydrothorax, especially when refractory to medical treatment, poses a challenging management dilemma. An early recognition and familiarity with available treatment modalities is crucial to effectively manage this exigent complication of cirrhosis.
Abbreviations used:
ESLD-End stage liver disease
TIPS-Transjugular intrahepatic porto-systemic shunt
VATS-Video-assisted thoracoscopy
SBEM-Spontaneous bacterial empyema
SBP-Spontaneous bacterial peritonitis
CPAP-Continuous positive airway pressure
LTx-Liver transplantation
IntroductionHepatic hydrothorax is defined as the presence of pleural fluid (usually greater than 500 cc) in a patient with cirrhosis in the absence of primary cardiac or pulmonary disease.1,2 This complication occurs in approximately 6-10% of patients with advanced cirrhosis.3 Although, more commonly associated with alcohol induced liver disease, cirrhosis from all causes can lead to the development of hepatic hydrothorax. The incidence of a pleural effusion is much higher with the concomitant presence of ascitic fluid. In one series of 330 patients with cirrhosis and ascites, 18 (6%) also had a pleural effusion.4 In another study, 6% of 200 patients with cirrhosis had a pleural effusion, but none of the 54 without ascites had demonstrable pleural fluid.5 Nevertheless, isolated rightsided hydrothorax can occur in a small number of patients.6,7
Unlike ascites where large volumes are generally tolerated due to the capacitance of peritoneal cavity, small volumes (1-2 L) of fluid within the pleural space can lead to significant symptoms. The effusions may affect either single or both pleural spaces, but there is predilection for the right hemithorax. Of interest, regarding the reported cases of hepatic hydrothorax, 85% have been right sided, 13% left-sided and 2 % bilateral.8
Hepatic hydrothorax, hepatopulmonary syndrome and pulmonary hypertension have been recognized as major pulmonary manifestations of cirrhosis. In this review, we present current understanding of patho-physiology, clinical manifestations, diagnosis and related complications of hepatic hydrothorax, and discuss therapeutic options available for this disorder.
PathophysiologyPleural effusion develops when rate of fluid accumulation exceeds its removal. Since its first description by Laennec in early 19th century, several mechanisms have been postulated (Table 1) for the development of hydrothorax in patients with cirrhosis.1,5,9-12 Regarding the mechanisms postulated; the direct passage of peritoneal fluid via diaphragmatic defects appears more plausible in explaining most cases of hepatic hydrothorax.3,13 This passage of fluid from peritoneal to pleural space has been demonstrated by various methods. In fact, Emerson in 1955 was the first to describe such a defect (post-mortem) in a patient with hepatic hydrothorax.9 These defects can be demonstrated not only grossly, but also microscopically in these patients. Lieberman and coworkers introduced CO2 into the peritoneal cavity of patients with hepatic hydrothorax.4 A pneumothorax indicative of a diaphragmatic defect was apparent in these patients on chest radiographs, taken within 48 hours. These defects may also be visualized using thoracoscopy.4,13,14 Intraperitoneal injection of methylene blue can be used intraoperatively to demonstrate and localize the defect(s). Furthermore, scintigraphic studies using intraperitoneal instillation of 99mTc-human serum albumin or 99mTc-sulphor-colloid have demonstrated transfer into the pleural cavity minutes to hours after administration.15,16 The movement of radioisotope is unidirectional towards the pleural cavity due to negative intrathoracic pressure compared to increased intra-abdominal pressure. These anatomic abnormalities permitting the passage of fluids have also been demonstrated with other imaging techniques.17
Proposed mechanisms for the development of hepatic hydrothorax.
| • Azygous vein hypertension causing formation of collateral anastomoses between portal and azygous systems.1 |
| • Transfer of peritoneal fluid into the pleural space via diaphragmatic defects.9 |
| • Passage of fluid from peritoneal to the pleural space via trans-diaphragamtic lymphatics.5,10 |
| • Hypoalbuminemia resulting in decreased colloid osmotic pressure.11 |
| • Lymphatic leakage from the thoracic duct.12 |
Microscopic examinations of these defects have revealed discontinuities or gaps in the collagen bundles that make up the tendonous portion of the diaphragm.4When ascitic fluid collects within the peritoneal cavity, it raises the intra-abdominal pressure and tends to stretch the diaphragm; thereby, creating or enlarging these microscopic defects. Increase in abdominal pressure (as a result of ascites) can result in herniation of peritoneum through these gaps in the pleural cavity. This leads to the formation of pleuro-peritoneal blebs. These blebs are typically less than 1 cm in diameter and tend to rupture, thus, providing free communication between the peritoneal and pleural cavities. These blebs tend to occur more commonly in the right hemidiaphragm. For reasons that are poorly understood but may be related to embryonic development, the left hemidiaphragm is more muscular and relatively resistant to blebs formation.8
Clinical presentationThe clinical presentation is usually dominated by signs and symptoms of cirrhosis and its complications, mainly ascites. Moreover, a pleural effusion may simply be an incidental finding on a chest radiograph performed for unrelated reasons. However, a small subset of cirrhotic patients do present primarily with pulmonary complaints related to hydrothorax.18 These symptoms may include dyspnea, non-productive cough, pleuritic chest pain or fatigue related to hypoxemia. Severe dyspnea and potential respiratory compromise can occur with large pleural effusions. Furthermore, these large effusions have the potential of causing cardiac tamponade with profound systemic hypotension that may require immediate intervention.19
DiagnosisClinical suspicionDiagnosis of hepatic hydrothorax can typically be made on clinical grounds. For example, a patient with established cirrhosis and ascites who is found to have a right-sided pleural effusion most likely has hepatic hydrothorax. However, patients can also present with a pleural effusion in the absence of a known diagnosis of cirrhosis and/or history of ascites. Others still, may have fever, respiratory symptoms or left-sided pleural effusion, suggesting an etiology other than the hepatic hydrothorax or the presence of spontaneous bacterial empyema (SBEM) (a poorly described complication which will be described later). Therefore, a diagnostic thoracentesis is mandatory in all patients with pleural effusions for two main reasons; first, to exclude the presence of an infection, and second to rule out an alternative diagnosis.
Pleural fluid analysisCauses of pleural effusions are numerous, and sometimes, in a cirrhotic patient cannot be readily distinguished from other etiologies besides portal hypertension. A nicely done study from Barcelona20 confirmed that many end-stage liver disease patients with pleural effusions do not have hepatic hydrothorax; as 18/60 (30%) patients upon thoracentesis yielded a diagnosis other than hepatic hydrothorax including, SBEM, tuberculosis, adenocarcinoma, parapneumonic empyema and undiagnosed exudates. Other investigators also suggest that both thoracentesis and paracentesis should be performed to ascertain that both fluids are similar in character.3 Although, there are no absolute contraindications to thoracentesis, increased caution is warranted in the presence of anticoagulation or a bleeding diathesis with a PT or PTT greater than twice the midpoint of the normal range, a platelet count less than 25,000/mm3 or a serum creatinine concentration greater than 6 mg/dL. Despite this caution, there is good safety profile for both paracentesis and thoracentesis in those with mild coagulation abnormalities.20-22 However, in a subset of patients with severe renal failure (creatinine levels 6-14 mg/dL); the risk of average hemoglobin loss was seven-fold higher than their counterparts.21
The composition of pleural fluid from hepatic hydrothorax, as expected, is similar to that of ascitic fluid given the etiology of these effusions.2 Pleural effusions associated with portal hypertension are always transudative.8 This same fact (shown in Table II) was affirmed by Light and colleagues.23 However, ascitic and pleural fluid analysis may not be completely identical, probably due to greater efficacy of water absorption by the pleural surface. In general, the cell count is low; total protein, albumin, cholesterol and total lipid levels may be marginally higher in the pleural fluid compared to ascitic fluid.24The serum-to-pleural fluid albumin gradient is usually greater than 1.1 g/dL, similar to the one resulting from portal hypertension, although, this has not been studied extensively.
Clinical and lab features of hepatic hydrothorax.
| Location |
| • Right sided (85%) |
| • Left sided (13%) |
| • Bilateral (2%) |
| No pericardial effusion |
| Laboratory features |
| • Cell count < 500 cells/mm3 |
| • Total protein concentration < 2.5 g/dL |
| • Total protein pleural fluid to serum ratio < 0.5 |
| • Lactate dehydrogenase pleural fluid to serum ratio < 0.5 |
| • Serum to pleural fluid albumin gradient > 1.1 g/dL |
| • Pleural fluid amylase concentration < serum amylase concentration |
| • pH 7.40 to 7.55 |
Spontaneous bacterial empyema(SBEM) is defined as an infection of a pre-existing pleural effusion (hydrothorax) in a patient with cirrhosis.25,26 Its estimated incidence is around 13% (similar to the incidence reported for spontaneous bacterial peritonitis; SBP) in cirrhotic patients with ascites.25,27 The pathogenesis of SBEM is also similar to that of SBP. The diagnosis of SBEM is established if the pleural fluid (PF) cultures are positive and a polymorphonuclear (PMN) count is > 250 cells/uL. In patients with negative cultures (and compatible clinical course) the diagnosis is made with a pleural fluid PMN count > 500 cells/uL and by excluding a parapneumonic infection.26 The microorganisms responsible for SBEM (E. coli, Klebsiella, Streptococcus and Enterococcus species) appear similar to that of SBP.25 Sese et al demonstrated that patients with hepatic hydrothorax had lower opsonic activity and complement (C3) levels than their counterparts with non-hepatic pleural effusions.28 In addition, all patients who developed SBEM had lower levels of pleural fluid C3 and total protein levels, and had a higher Child-Pugh score than those who did not develop SBEM.
SBEM can present in various ways including as that seen with SBP (abdominal pain), symptoms may be localized to the chest cavity (dyspnea or pleuritic chest pain), or may be systemic in nature (fever, shock or encephalopathy). Similar to hepatic hydrothorax, SBEM can occur in the absence of ascites. Interestingly, up to 40% of SBEM cases may not be associated with SBP.25The treatment of SBEM is similar to that of SBP (intravenous 3rd generation cephalosporins) for 7 to 10 days.25We prefer cefotaxime at a dose of 2gm Q8h due to its very good published results in cirrhotic patients.29,30However, despite treatment, mortality remains high at approximately 20%.25
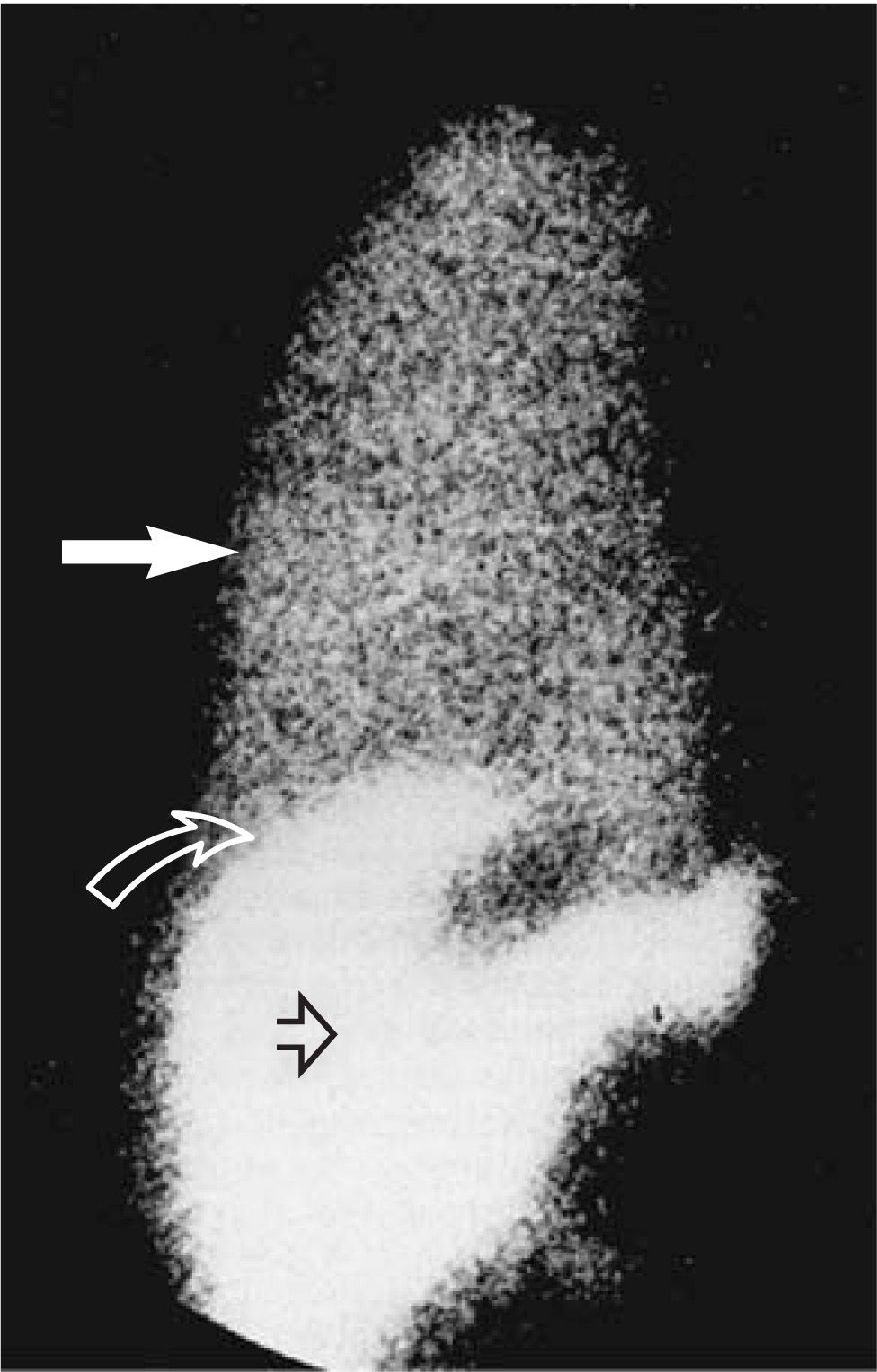
Radiological studiesNuclear scans can be performed to establish the diagnosis of hepatic hydrothorax with fairly high accuracy. Intra-peritoneal administration of 99mTc-human serum albumin or 99mTc-sulphur colloid could be used to demonstrate the communication between the peritoneal and pleural space as shown in Figure 1.31 The migration of radioisotopes from the peritoneal cavity into the pleural space confirms the presence of a communication and the effusion.15,31 This diagnostic modality can also be used in the absence of ascites via ultrasound guided administration of the isotope (in 500 cc of saline) into the peritoneal cavity.6 The lack of radioisotope to localize in the pleural space should raise concern for a primary cardiopulmonary process responsible for patient’s pleural effusion. Although, this test has been considered the «gold standard» for identification of hepatic hydrothorax due to very high specificity (up to 100%), its sensitivity remains modest ≈ 71%. The sensitivity of the test can be greatly improved (up to 100%) by performing a thoracentesis prior to administration of radioisotopes in order to reduce pleural pressure.32 Various other investigators have used modalities similar to radioisotopes; such as intraperitoneal injections of dyes and air to diagnose hepatic hydrothorax.4,3
Anterior view of the chest and upper abdomen after injection of 99mTc-sulphur colloid into the peritoneal cavity and showing passage of the radioisotope into the right pleural space. White solid arrow-isotope in right pleural cavity; curved open arrow-right hemidiaphragm; and straight open arrow-isotope in peritoneal cavity. (Adopted from Bhattacharay, et al.31)
Recent advances in radiological imaging (ultrasound, CT scans and MRI) have enabled investigators to examine in detail the diaphragmatic defects responsible for the development of hepatic hydrothorax.17,33
Invasive techniquesThoracoscopy can be an alternative diagnostic option to directly visualize the underlying diaphragmatic defects. The concomitant administration of dyes in the peritoneal cavity may increase the probability of locating such defects, in case they are not readily apparent on thoracoscopy.13
ManagementSince hepatic hydrothorax and ascites share the same pathophysiological mechanisms; understandably, therapy is directed towards the underlying mechanisms of fluid accumulation, namely, sodium retention and sinusoidal hypertension. Management can involve dietary, pharmacologic and radiological interventions. In addition, surgical approaches aimed at repairing the diaphragmatic defects responsible for pleural fluid accumulation can be considered in selective patients with refractory hydrothorax. However, a majority of these patients with hepatic hydrothorax have advanced liver disease and may be potential candidates for orthotopic liver transplantation; the only effective cure to date. Thus, the aim of therapy in such patients should be relief of symptoms and prevention of further complications until transplantation can be performed. An algorithm for the management of these patients is illustrated in figure 2, and the various potential therapeutic options available to date are discussed in detail below.
Medical managementSimilar to the therapy of ascites, obtaining a negative sodium balance is the primary goal of dietary and pharmacologic management.34 Dietary restriction of sodium intake to 2 g/d (88mEq/d) is the simplest manner by which to achieve a negative sodium balance. In fact, a low sodium diet alone has shown to be effective in eliminating ascites in 10-15% of patients.35 However, most patients with ascites, and almost all patients with hepatic hydrothorax require the addition of diuretics (spironolactone and/or furosemide). These diuretics are typically maintained at a ratio of 10:4 (spironolactone 100 mg: furosemide 40 mg), and dosages are increased as needed to attain a goal of producing renal excretion of at least 120mEq of sodium per day.36 However, in many patients these goals are not achieved due to diuretic induced electrolyte imbalances, renal impairment or precipitation of encephalopathy. When diuretic therapy is deemed not helpful, patients should be considered for orthotopic liver transplantation.
Other investigational agents such as terlipressin, octreotide and midodrine have been used, mainly in case reports, to treat hepatic hydrothorax with moderate benefit.37-39 It is postulated that these agents will reduce splanchnic blood flow and hence decrease peritoneal and pleural fluid accumulation. Unfortunately, at present there is not enough evidence to recommend routine use of these agents.
ThoracentesisThoracentesis is a simple and relatively safe procedure that can be used not only for diagnostic but therapeutic purposes as well. Thoracentesis can be performed in patients with dyspnea due to hepatic hydrothorax for immediate relief of symptoms. In patients with both hepatic hydrothorax and massive ascites, it is recommended to drain the ascites prior to performing a thoracentesis.2 Furthermore, generally, no more than 2 liters of fluid should be removed during the first therapeutic thoracentesis, in order to minimize the risk of unilateral pulmonary edema and/or hypotension.8,40 The effect of routine use of albumin infusion in conjunction with thoracentesis has not been established. Although, in one study investigators routinely infused albumin (5 g per each liter of fluid removed); no further conclusions were drawn about its effect on the patients outcome.20 The major risk of thoracentesis appears to be the development of pneumothorax. In the above study, investigators also showed that diagnostic thoracentesis carried a low risk (1%) of pneumothorax, however; the incidence rose to 9% after a therapeutic procedure. Despite its relative safety, when thoracentesis is required too frequently (< every 2-3 weeks) in patients on maximal sodium restriction and optimal diuretics, alternative treatment options must be considered.8
Radiologic interventions: Transjugular intrahepatic portosystemic shunts (TIPS)Over the past decade, TIPS has been used more often in patients who have failed medical management or those who require frequent thoracentesis.41-47 By creating a nonselective side-to-side porto-systemic shunt, TIPS decreases portal pressures, and addresses the sinusoidal hypertension that leads to ascites formation-an essential step for pleural fluid accumulation.48 The use of TIPS for hepatic hydrothorax was first described in a case series by Strauss et al41 in 1994. In this study, 5 patients with Child class C cirrhosis underwent TIPS placement. Two of these patients did not require further thoracentesis, while in the remaining 3, further fluid removal was required due to stent occlusion. However, once stent patency was restored, the need for repeat thoracentesis was eliminated. A larger study by Gordon et al, evaluated 24 Child class B and C cirrhosis patients following TIPS placement.43 Fourteen of twenty four (58.3%) had complete resolution of symptoms following TIPS and did not require further thoracentesis; while another 5 (20.8%) required fewer number of thoracentesis. Despite this superior efficacy, 6 patients (25%) died of either post-procedure complications (1/6) or liver failure (5/6), and 9 (37.5%) developed transient hepatic encephalopathy. Other groups have reported even higher initial success rates (≈ 82%) with TIPS, though; less stringent criteria (such as decrease in effusion size or reduced need for thoracentesis) were used in these studies.46 But again, despite symptomatic improvement in many patients, TIPS unfortunately was associated with complications and did not improve the overall prognosis of these patients. A recent study of 28 patients shows that severity of liver dysfunction is directly related to non responsiveness and higher one year mortality after TIPS placement for refractory HH.49Thus, because TIPS is associated with many potential risks, it should be considered in selected patients who re-accumulate their effusions rapidly (despite medical treatment) with a Child-Pugh score of less than 10, are younger than 60, and do not have hepatic encephalopathy or severe pulmonary hypertension. The factors associated with poor prognosis after TIPS placement are illustrated in Table III.50 Since a high mortality is anticipated in this patient population in the absence of liver transplantation, it is not surprising that only a third of patients are expected to live and be relapse free at 1 year after the TIPS placement.46,50
Surgical interventionsThe surgical approaches for the management of hepatic hydrothorax have included; tube thoracostomy with chemical pleurodesis, thoracoscopy to repair diaphragmatic defects with/without pleurodesis and placement of peritoneovenous shunts.
Pleurodesis: Falchuk et al first described the use of tetracycline induced pleurodesis for 2 patients with recurrent hepatic hydrothorax.51 One patient remained free of effusion at 6-month follow up while the other died of variceal hemorrhage 3 weeks after the pleurodesis. Chemical pleurodesis is not always successful and does carry a modest risk of complications including fever, chest pain, empyema, incomplete re-expansion, pneumonia, and wound infection among others.52 Ikard and colleagues53 reported two unsuccessful attempts with tetracycline pleurodesis, attributed to large flow of ascites across the diaphragm, resulting in a thoracic cavity that could not be kept empty enough to allow pleural coaptation and subsequent chemical pleurodesis. Because of its low success rate and moderate degree of complications, pleurodesis by itself is rarely performed and typically reserved for patients in whom no other options exist. However, the use of continuous positive airway pressure (CPAP) appears effective in keeping the pleural cavity dry after chemical pleurodesis.54 It is postulated that CPAP will decrease the negative pleural pressure and thus prevent the shift of fluid form the peritoneal to the pleural space. Furthermore, Takahashi55 has shown dramatic improvement in their patient with refractory hepatic hydrothorax using only nasal CPAP, but further studies are needed before this can be routinely recommended.
Chest tube placement: Runyon et al reported their experience with 2 patients with ascites and persistent rightsided pleural effusions, in whom chest tube placement led to massive fluid shifts, protein and electrolyte depletion and eventually death of both patients.56 At present, chest tube insertion is considered a relative contraindication for the treatment of hepatic hydrothorax.
Repair of diaphragmatic defects: Thoracoscopy to repair diaphragmatic defects with/without sclerosing the pleural membranes may be an alternative in patients with refractory hepatic hydrothorax who are deemed not candidates for TIPS. Thoracoscopy appears to be more likely to succeed if diaphragmatic defects can be identified.57 Mouroux et al employed video-assisted thoracoscopy (VATS) to close large defects using sutures and biologic glue in combination with talc pleurodesis in 8 patients.13 None of the patients (6/8) with repaired defects developed recurrent hydrothorax despite the recurrence of ascites. In another study, de Campos et al performed 21 VATS, with talc pleurodesis in 18 patients-all with hepatic hydrothorax.52 The overall success rate was 48% despite thoracoscopy revealing defects in only 5 of the 18 patients. However, a higher mortality was noted in this study with 7 of 18 patients dying between 5 and 40 days of follow-up. In contrast, two other studies (15 and 41 patients each) showed almost 75% success rate with VATS assisted talc pleurodesis without resorting to diaphragmatic repairs,58,59 though, repeat procedure was required in many patients.59 Thoracoscopic repair of diaphragmatic defects using pleural flap or mesh onlay reinforcement also shows good early results in patients with refractory hydrothorax.60 In brief, this procedure (and its variations) may be considered a palliative alternative not only to patients requiring frequent thoracentesis, but also an alternative to TIPS. Furthermore, it may also be used as a bridge to liver transplantation, if larger randomized trials confirm the previous results and show a lower mortality rate.
Peritoneo-venous shunts: A peritoneo-venous shunt (Le Veen shunt) to divert ascitic fluid has been used in refractory cases. However, after the initial efficacy, in most cases, the shunt is rendered ineffective over time as the intrathoracic pressure is lower than the central venous pressure resulting in fluid flow towards the pleural space.53 Due to these inconsistent results and frequent complications associated with LeVeen shunt (infection, coagulopathy and bleeding in compromised host), this procedure has become almost obsolete.6
Liver transplantation: When all other therapies fail, liver transplantation is the only option available; which fortunately is curative for most patients with this complication. The short-term complications and long-term prognosis in patients undergoing liver transplantation for refractory hepatic hydrothorax appears similar to other groups.61
ConclusionHepatic hydrothorax is a devastating complication of end-stage liver disease, and in fact, is not that uncommon as previously thought. Its management is challenging and frequently associated with poor outcomes in most cases. Non-the-less an early diagnosis is of vital importance to establish an appropriate management plan. When other measures fail, liver transplantation remains the treatment of choice. Both TIPS and possibly VATS assisted diaphragm repair, though, temporizing measures, are perhaps the best available “bridging” to liver transplantation in selected patients with refractory hepatic hydrothorax.