Introduction. Based on very limited data, it has been recently suggested that hepatitis B virus infection can play significant roles in post transplantation lymphoproliferative disorders. In the current study pooling data of PTLD in HBV positive liver recipients gathered from the existing literature, we sought to analyze and compare characteristics, behavior and prognosis of PTLD arising in HBV positive liver graft recipients.
Methods. A comprehensive search for the available data though PubMed and Google Scholar for reports of PTLD and HBV infection in liver recipients was conducted. Data of 18 different studies were pooled and analyzed.
Results. Liver recipients with HBV positive-PTLD were comparable to their HBV negative counterparts in gender, age at transplantation, time from transplantation to PTLD development, lymphoma cell type, histopathology of lesions, remission episodes, mortality rate, multi-organ involvement, and disseminated PTLD (p > 0.1 for all). HBV positive PTLD patients were significantly less likely to complicate spleen (0 vs. 23%, respectively; p = 0.015). Survival of the two patient groups were comparable (p = 0.8).
Conclusion. HBV infection has no significant impact on inducing some distinct types of PTLD and represents no survival effect in PTLD setting. Future prospective studies are needed for confirming our findings.
During the past two decades, due to enhancements in preventive and therapeutic methods, the organ transplantation practice has witnessed substantial improvements in the outcome of organ recipients, especially within their early post transplantation period.1 These advantages have resulted in an increase in the survival of transplant patients; and expression of some newly emerged complications adversely affecting the outcome of the practice.
Lymphoproliferative disorders after solid organ transplantation (PTLD) is one of the most prevalent malignancies that develops in organ recipients, and remains a challenging diagnostic and therapeutic problem in the context of transplantation. PTLD is characterized by lymphoid proliferation of B- or T-cell origin and was first reported by Penn, et al.,2 in 1969, in a patient who had undergone living related kidney transplantation. Since then, several reports have been published indicating a high incidence of PTLD among recipients of all types of organs, including liver. The incidence of lymphomas after transplantation is quite higher than that in the general population.3–8 Reported reasons for this high incidence include greater levels of immunosuppression, antibody induction therapy including OKT3, anti-lymphoblast globulin (ALG) and antithymocyte globulin (ATG) and viral infections especially Epstein-Barr virus (EBV) infection.6–8
Evidence suggests that hepatitis B virus (HBV) can act as a lymphotropic virus in the normal population and has been implicated as a risk factor for the development lymphoproliferative disorders.9,10 The prevalence of HBV among non-Hodgkin’s lymphoma (NHL) patients has been reported high in hepatitis B endemic areas.11 Moreover, it is demonstrated that chronic HBV infection is associated with a higher incidence of NHL in non-transplant population9 and patients with B cell NHL reportedly represent higher prevalence of HBV infec-tion.10 The first evidence indicating a potential association between HBV infection and PTLD development has been published in 2008 as a series of three;12 and one year later in 2009, Zhang, et al.13 published another report confirming a possible association between HBV infection and PTLD. Taken together, existing data suggests that HBV can play a pathogenic role in inducing NHL both in the general population and organ transplant patients.
Although there are some evidences indicating the impact of HBV on the development of PTLD, however as mentioned above, the existing data are based on very limited number of case reports or small series; moreover, there is no mention whether PTLD arising in HBV positive patients represents any special features and prognosis. Due to the limited number of reports, there is almost no data on any potential specificity for HBV induced PTLD. In fact, such cases are only included in bigger series and have not received enough attention. Pooling data of PTLD in HBV positive liver recipients from the existing literature, we sought to analyze and compare characteristics, behavior and prognosis of PTLD arising in HBV positive liver graft recipients.
Materials and MethodsApproach to the studyWe conducted a comprehensive search for the available data though PubMed and Google Scholar for reports of PTLD and HBV infection. Search terms used were:
- •
Lymphoproliferative disorders + transplantation + liver + hepatitis B virus.
- •
Lymphoproliferative disorders + transplantation + liver + HBV.
- •
PTLD + liver + hepatitis B virus.
- •
PTLD + liver + HBV.
In cases where we were not able to obtain the full text of the article, emails were sent to correspondent authors requesting the article. Of the full texts obtained, we only included studies in which data on each patient was presented separately. To minimize selection bias, HBV negative PTLD controls were included only from the same studies, cases were enrolled from. A standard questionnaire was developed to collect data from different published studies. The time between transplantation and PTLD onset was defined as the period between the engraftment and the first signs of PTLD or diagnosis, depending on the study’s approach.
Study populationEighteen international published studies12–29 were found that met our criteria. A total of 151 cases of PTLD liver transplant patients were included in the analysis; of whom 42 (28%) were HBV positive-PTLD and the remaining 109 (72%) patients had developed HBV negative-PTLD. EBV status was documented in 107 (71%) patients, of whom 73 (68%) were reported positive.
Because methodologies differed among the published studies, not all our measures were available for all patients. We recorded disseminated PTLD when it was reported by the study authors or if at least three different organs were involved by the PTLD (different lymph node areas were excluded from analysis due to lack of knowledge on how to categorize; unless they were concomitant with other organs involvements; or other authors specifically presented them as having disseminated disease). According to the abovementioned, disseminated disease was reported for 20 patients (23%; 64 missing data). Multi-organ involvement, defined as involvement of more than one organ (the second organ could be a lymphatic region), was available in 36 patients (40%; 61 missing data).
At PTLD onset, all patients were under immunosuppressive regimens consisting of varying combinations of azathioprine, prednisone, cyclosporine, mycophenolate mofetil, ATG/ALG and OKT3. A rather uniform approach was used to manage most of the included PTLD liver recipients. On diagnosis of PTLD, the first step in almost all reports was to decrease or discontinue immunosuppressive therapy; various regimens of chemotherapy with or without surgical interventions were also used for some patients.
Response to treatmentWe defined response to treatment as any favorable change both in PTLD measures as well as the patient’s clinical condition. Data on response to treatment was reported for 38 patients (25%), of whom 28 (74%) responded to treatment. To create a common standard across the studies, we defined a remission episode as when a patient was alive 24 months after PTLD onset (because all reported cases meeting this criterion had at least one confirmed remission episode) and no remission as when a patient died within the first month after PTLD onset (because there were no patients dying at the first post-transplant month that was reported to have any remission episodes). According to these criteria, data on remission was available for 70 patients (47%), of whom 58 (83%) had at least one response to treatment, irrespective of their future disease course. Data on mortality was available for 100 patients (67%), of whom 39 (39%) died. We defined death due to PTLD when the authors stated it, death was within 6 months after onset, or death was reported to be due to PTLD treatment complications. Based on these criteria, 17 patients (44% of reported deaths, 29% of patients for whom mortality data was reported) died due to PTLD.
Statistical analysisSPSS v.13.0 software was used for data analyses. Statistical comparisons between patient subgroups were performed using chi-square and Fisher’s exact tests for proportions, and the Student’s t-test for continuous data. Survival analysis was done with life tables, Kaplan-Meier method and log-rank test. A p-value of 0.05 was taken as the threshold for significance.
ResultsOverall, 151 cases of PTLD were found. There were 96 (64%) male and 51 (36%) female patients (4 missing data). Mean age at onset was 45.4 ± 15.0 years. The mean interval between transplantation and the onset of PTLD was 38.0 ± 37.5 months and the mean follow up time after onset of PTLD was 23.5 ± 31.2 months.
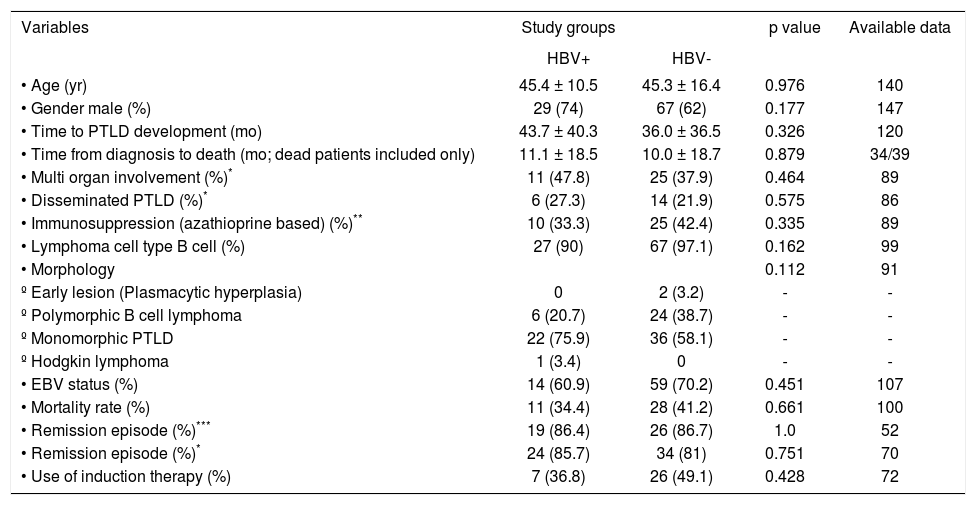
Characteristics of PTLD patients with and without HBV infection are summarized in table 1. Chi-square test showed that HBV positive-PTLD patients were comparable to HBV negative-PTLD in gender (p = 0.18), age at transplantation (p = 0.976), time from transplantation to PTLD development (p = 0.326), lymphoma cell type (p = 0.16), remission (p = 0.75), mortality rate (p = 0.66), multi-organ involvement (based on our definition; p = 0.46); and disseminated PTLD (based on our definition; p = 0.57). Histopathological evaluations were also not significantly different between the two groups (p = 0.11).
Characteristics of liver transplant recipients.
| Variables | Study groups | p value | Available data | |
|---|---|---|---|---|
| HBV+ | HBV- | |||
| • Age (yr) | 45.4 ± 10.5 | 45.3 ± 16.4 | 0.976 | 140 |
| • Gender male (%) | 29 (74) | 67 (62) | 0.177 | 147 |
| • Time to PTLD development (mo) | 43.7 ± 40.3 | 36.0 ± 36.5 | 0.326 | 120 |
| • Time from diagnosis to death (mo; dead patients included only) | 11.1 ± 18.5 | 10.0 ± 18.7 | 0.879 | 34/39 |
| • Multi organ involvement (%)* | 11 (47.8) | 25 (37.9) | 0.464 | 89 |
| • Disseminated PTLD (%)* | 6 (27.3) | 14 (21.9) | 0.575 | 86 |
| • Immunosuppression (azathioprine based) (%)** | 10 (33.3) | 25 (42.4) | 0.335 | 89 |
| • Lymphoma cell type B cell (%) | 27 (90) | 67 (97.1) | 0.162 | 99 |
| • Morphology | 0.112 | 91 | ||
| º Early lesion (Plasmacytic hyperplasia) | 0 | 2 (3.2) | - | - |
| º Polymorphic B cell lymphoma | 6 (20.7) | 24 (38.7) | - | - |
| º Monomorphic PTLD | 22 (75.9) | 36 (58.1) | - | - |
| º Hodgkin lymphoma | 1 (3.4) | 0 | - | - |
| • EBV status (%) | 14 (60.9) | 59 (70.2) | 0.451 | 107 |
| • Mortality rate (%) | 11 (34.4) | 28 (41.2) | 0.661 | 100 |
| • Remission episode (%)*** | 19 (86.4) | 26 (86.7) | 1.0 | 52 |
| • Remission episode (%)* | 24 (85.7) | 34 (81) | 0.751 | 70 |
| • Use of induction therapy (%) | 7 (36.8) | 26 (49.1) | 0.428 | 72 |
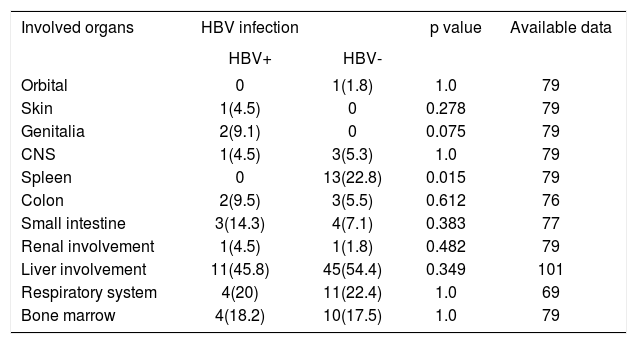
Table 2 compares HBV-positive vs. HBV-negative PTLD respecting organ involvements by the PTLD. HBV positive PTLD patients were significantly less likely to complicate spleen (0 vs. 23%, respectively; p = 0.015); but no other differences were seen for the two patient groups. At the last follow up, 39 patients (39% of reported; 50 missing data) were dead. Using death by any cause as the final outcome, logrank test did not show any difference between the two groups in survival (p = 0.78) (Figure 1). Nor was any difference seen between the two groups when only death due to PTLD (based on our definition) was used as the final outcome (p = 0.36). Five-year survival rate for HBV-positive PTLD patients was 64% compared to 56% for HBV-negative PTLD patients.
Frequency of involved organs in 168 liver transplant recipients with early or late onset PTLD.
| Involved organs | HBV infection | p value | Available data | |
|---|---|---|---|---|
| HBV+ | HBV- | |||
| Orbital | 0 | 1(1.8) | 1.0 | 79 |
| Skin | 1(4.5) | 0 | 0.278 | 79 |
| Genitalia | 2(9.1) | 0 | 0.075 | 79 |
| CNS | 1(4.5) | 3(5.3) | 1.0 | 79 |
| Spleen | 0 | 13(22.8) | 0.015 | 79 |
| Colon | 2(9.5) | 3(5.5) | 0.612 | 76 |
| Small intestine | 3(14.3) | 4(7.1) | 0.383 | 77 |
| Renal involvement | 1(4.5) | 1(1.8) | 0.482 | 79 |
| Liver involvement | 11(45.8) | 45(54.4) | 0.349 | 101 |
| Respiratory system | 4(20) | 11(22.4) | 1.0 | 69 |
| Bone marrow | 4(18.2) | 10(17.5) | 1.0 | 79 |
PTLD is a potentially fatal complication of transplantation in all types of organ recipients. The incidence, features and prognosis of PTLD varies between different organ transplant patients due to several interfering factors including the transplanted organ, doses of immunosuppression used, the use of antibody induction, and viral infections most notably EBV infection.31–33 In non-transplant setting, hepatophil viruses are shown to have provocative roles in inducing lymphoproliferative disorders. Recently, it has been suggested that HBV reactivation after transplantation is a risk factor for the development of PTLD.12,13 One reason for this risk enhancement is reportedly the immunosuppressive treatment in these patients due to preventing rejection episodes leading to reactivation of chronic infections including HBV.
In this study of international data, we gathered the existing data from HBV infected liver transplant recipients who had developed PTLD in their disease course to analyze and search any potential associations between HBV infection in PTLD patients and PTLD features, behavior and prognosis in liver graft recipients. The unexpected finding of this study is that we found almost no difference between HBV positive and negative PTLD patients. Although, there might be some criticisms on the methodology of our study which would be later discussed, we think that this finding is not only due to the limited number of included patients because to the best of our knowledge, there is no other study that has investigated even a comparable number of HBV positive patients in PTLD setting.
Moreover, this topic has not been previously discussed except for a series of 3 cases12 and one case re-port13 and they have provided the first evidence for a potential association between HBV infection and PTLD. On the other hand, methodology of our study which reviews and gathers data from different reports, despite some disadvantages, can be used precisely in several cases. For example, if HBV infection could have a provocative role for development of PTLD, at least we should expect a shorter time from transplantation to PTLD development in HBV positive PTLD patients compared to HBV negative controls; as we can observe it in EBV positive patients in transplantation setting.32,33 Moreover, no disparities were also found in the behavior of the PTLD between HBV-positive and -negative liver recipients including remission rates and survival rates. This finding suggests that, even if HBV has any role in the development of the PTLD, it does not induce a more malevolent or fatal form of the disease than that in non-infected subjects. An interesting observation was that HBV positivity was not associated with a hepatic localization of the PTLD; the only disparity found between the two groups was a less likelihood of HBV positive PTLD lesions to complicate the spleen. In fact, none of HBV positive subjects represented a spleen complication while 23% of HBV negative controls developed splenal involvement by PTLD. Although we have no explanation for this observation, the considerable difference observed cannot be simply disregarded as an incidence.
To exclude any simultaneous messing effects of hepatitis C virus infection on our results (5 cases), we reanalyzed our data after excluding these cases. No change has been observed after the adjustment (data not shown). Rituximab has also been reported to reactivate HBV infection and as a consequence it might play as a stimulating factor for the development of lymphoma.34–36 Unfortunately, we only had data of our patients after the development of the PTLD; so, we were not able to investigate a potential impact of rituximab on HBV-associated PTLD.
Nevertheless, we analyzed data of our HBV positive PTLD liver recipients with respect to having (10; 24%) or not having (32; 76%) a history of rituximab treatment after PTLD emergence. We found that patients under rituximab treatment significantly had higher rate of multi-organ disease (data not shown); but it probably was the rationale for which rituximab was employed for these patients. Moreover, we found no survival difference between HBV positive patients who had or had not used rituximab; this can confirm current study’s findings that HBV has no prominent role in the prognosis of the PTLD; since despite its HBV activating role, rituximab had no deteriorating impact on the outcome of HBV positive PTLD patients. Due to the very limited number of cases with a history of antiviral therapy, we were not able to evaluate potential impact of antiviral agents on the PTLD outcome.
Potential criticisms may arise over our study. First, our study population was gathered from different reports with inconsistent approaches. We also believe that this is the unique major limitation for this study leading to substantial missing data for some of study variables and thus, decreasing the power of our analyses. This limitation was most prominent for special data that are not typically included in reports on PTLD patients. Another limitation due to the inconsistencies available between the included studies was that results of different studies were not presented in the same way. For example, report of any response to treatment was presented very dissimilarly in different studies; while in one study partial and complete remission was used to translate the results, in another only “response to treatment” was used and in some others no specific terminology was employed. So, we ought to invent new methods to cumulate the existing data for analysis.
We conclude that, HBV infection has no significant impact on inducing some distinct types of PTLD and represents no survival effect in PTLD setting. Future prospective studies are needed for confirming our findings.