HCV-infected immigrants contribute to the total prevalence in Canada and other developed nations. Little is known about engagement in care, access to service, and treatment outcomes in recipients of Direct Acting Antiviral (DAA) HCV therapies among immigrants living with HCV.
Material and methodsHCV patients assessed at The Ottawa Hospital Viral Hepatitis Clinic between 2000-2016 were identified. Immigration history, race, socioeconomic status, HCV work-up, treatment and outcome data were evaluated. HCV fibrosis assessment, treatment and sustained virologic response (SVR) were compared using logistic regression.
Results2,335 HCV-infected patients were analyzed with 91% (2114) having data on immigration (23% immigrants). A median 16 years (Quartiles: 5, 29) passed from immigration to referral. Access to diagnostic procedures (Fibroscan/liver biopsy) was greater among immigrants compared to Canadian-born (78% vs. 68%, p = 0.001) and immigrants had an odds ratio of 1.72 (95% CI: 1.18-2.51) of receiving a FibroScan compared to Canadians after adjustment for demographic characteristics, HCV risk factors, and socioeconomic status. No differences in SVR were found between immigrants for IFN recipients. Among DAA recipients, rates of SVR were > 94% among all patients, 93% in Canadian-born and 98% among immigrants (p = 0.14).
ConclusionNearly 80% of immigrants in this setting had access to fibrosis assessment which was higher than Canadian-born patients. Under half of both groups had initiated HCV therapy. Delays in accessing HCV care represent a missed opportunity to engage, treat and cure HCV patients. HCV screening and health care engagement at the time of immigration would optimize HCV care and therapeutic outcomes.
The prevalence of chronic hepatitis C virus (HCV) infection in Canada is estimated to be between 0.6% and 0.7% of the population (221,000-246,000 individuals) with greater than 40% of these individuals being undiagnosed.1 HCV prevalence is higher among certain subpopulations including inmates, people who inject drugs and foreign-born populations.1 With more than 230,000 immigrants and refugees arriving per year between 2006-2011,2 the foreign-born represent a rapidly growing proportion of the Canadian population.3 In addition, following policy changes in 1960s, many more recent immigrants originate from HCV-endemic regions compared to previous generations of immigrants who arrived primarily from Western Europe.4,5 In the past three decades, an increasing proportion of immigrants are of Middle East/North African, South Asian, Chinese, and African-Caribbean Black descent, with a majority of immigrants settling in Ontario.2,6 HCV screening at the time of immigration is not mandatory. Consequently, many immigrants remain undiag-nosed with HCV infection for many years after immigration before referral to viral hepatitis clinics for management.
Studies of immigrant health in Canada have indicated that foreign-born populations report higher levels of self-assessed health and lower levels of chronic conditions compared to their Canadian-born counterparts.7 Despite an initial relative health advantage, immigrants often face a variety of barriers to optimal health care and treatment access including patient-physician communication and language discordance, racial, ethnic, or religious disparities, and inequalities arising from socioeconomic differences.8 Over time, such barriers may limit access to physician, preventive or diagnostic services, and may contribute to an observed convergence in health parameters between immigrants and native-born residents within 5-20 years of arrival. Preliminary evidence suggests that immigrant status may be a barrier to HCV investigation and treatment.9 However, it remains an important question as to whether immigration to Canada influences HCV management. Furthermore, the impact of new all oral based Direct Acting Antiviral (DAA) therapies on immigrant treatment outcomes is unstudied. We evaluated HCV management and antiviral treatment outcomes in Canadian immigrants and Canadian-born residents within the setting of an urban tertiary care hospital based multidisciplinary viral hepatitis program.
Material and MethodsStudy design and data collectionThe study was conducted utilizing a retrospective cohort of patients followed within The Ottawa Hospital Viral Hepatitis Program. The program serves a regional catchment area of 1.5 million individuals and has a multiracial, multicultural and multilingual population.9 The charts of all patients visiting the clinic aged 18 years and older, chronically infected with HCV (HCV RNA positive more than 6 months after initial exposure), and who provided appropriate consent were reviewed. The cohort contains data collected between June 2000 and June 2016. Data were abstracted from clinical records using standardized data collection forms and entered into an electronic database. The analytical sample was restricted to the most recent round of HCV therapy. The Ottawa Health Science Network Research Ethics Board reviewed and approved this study [REB# 2004-196].
OutcomesWe compared access to fibrosis assessment, HCV treatment initiation and treatment success according to immigrant status. Access to fibrosis assessment was evaluated by the proportion receiving liver biopsy and/or transient elas-tography (FibroScan). These procedures are considered standards of pre-HCV antiviral care. FibroScans have been offered at our site since November 2012. The proportion of patients initiating HCV antiviral therapy and the therapy composition [interferon/pegylated interferon (IFN) ± ribavirin (RBV) ± Direct-Acting Antiviral (DAA) based or IFN-free DAA regimen] was determined. Treatment success was defined as sustained virological response (SVR12) using a modified intent-to-treat analyses among patients with sufficient follow-up (HCV RNA negative 12 or more weeks following the completion of HCV antiviral therapy).
Independent variablesThe primary independent variables included patient birth location (foreign- vs. Canadian-born) and length of time since immigration. Immigration history was patient reported and verified by clinic staff. Additional groups for recent (< 15 years of residency in Canada) and long-term resident (≥ 15 years) immigrants were created to examine the influence of length of time since immigration. A 15 year stratification time was selected on the basis that this duration represents a point at which risk factor profiles generally converge with native born populations.7
Covariates included age, sex, race (White, Black, Asian, or Aboriginal), history of alcohol use, history of injection drug use, HIV co-infection, HCV genotype, baseline laboratory data (HCV RNA, alanine aminotransferase [ALT], aspartate aminotransferase [AST]), Fibroscan fibrosis score (kilopascals [kPa] and converted Metavir-stage), liver biopsy stage and grade (Metavir), and socioeconomic status (SES). SES data were linked using area-level neighbourhood profiles from the 2006 Census. Using postal codes, patients were geo-coded to their neighbourhood of residence (Census dissemination area) using the Postal Code Conversion File (PCCF) program developed by Statistics Canada.10 This program assigns patients to their local area based on census geography using full six-digit postal code. We considered quintiles of neighbourhood-based income (adjusted for household-size) and a binary indicator of whether the patient was from the lowest income quintile as the primary markers of SES in this study.11,12
Statistical analysesDescriptive analyses involved frequencies and proportions for categorical variables. Means and standard deviations (SD) or medians and 1st/3rd quartiles were calculated to demonstrate central tendency and dispersion in continuous measures. Statistical comparisons across immigrant status groups were made using χ2 tests for categorical variables and t-tests for continuous variables. Predictors of clinical care management for HCV and SVR-12 were assessed via logistic regression which included adjustment for covariates and potential confounders. Because of the large differences in rates of SVR between IFN- and DAA-based treatments, all analyses of SVR were stratified by type of therapy. Statistical analyses were conducted using Stata (version 14.1).13
ResultsPopulation characteristicsBetween the years 2000 to 2016, 2335 HCV-infected patients were identified in the HCV database. Of these, 2114 (91%) had data available on immigration (n = 494 immigrants, 23%) and 459/494 (93%) immigrants had data on length of time in Canada. The median length of time since immigration in this sample was 16 years (quartiles [5,29]). Immigrants were older at the time of initial assessment compared to those born in Canada (Table 1). A small number of immigrants (0.6%) who identified as Aboriginal originated from the United States.
Descriptive characteristics of chronic HCV infected patients among immigrants and those born in Canada in the Ottawa Viral Hepatitis Program 2000-2016.
| Overall | Immigrants | Canadian Born | |||||
|---|---|---|---|---|---|---|---|
| 1. Continuous variables | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | p-value |
| Age (y) | 2,114 | 47.2 (11.3) | 494 | 50.9 (13.6) | 1,620 | 46.1 (10.2) | < 0.001 |
| Viral load (IU/mL) | 2,029 3.8e + 06 (1.3e + 07) 475 3.9e + 06 (2.2e + 07) 1,554 3.8e + 06 (8.7e + 06) 0.915 | ||||||
| AST (IU/L) | 2,058 | 69.2 (75.1) | 483 | 64.7 (49.3) | 1,575 | 70.6 (81.3) | 0.134 |
| ALT (IU/L) | 2,073 | 96.5 (112.0) | 489 | 87.6 (78.6) | 1,584 | 99.3 (120.3) | 0.043 |
| Fibroscan score(kPa) 635 | 12.7 (13.2) | 176 | 12.5 (11.1) | 459 | 12.8 (14.0) | 0.831 | |
| Duration of HCV | 2,009 | 24.9 (13.6) | 469 | 32.2 (15.4) | 1,540 | 22.7 (12.1) | < 0.001 |
| infection (years) | |||||||
| 2. Categorical variables | n/N | % | n/N | % | n/N | % | p-value |
| Male | 1,407/2,114 | 66.6 | 274/494 | 55.5 | 1,133/1,620 | 69.9 | < 0.001 |
| Female | 707/2,114 | 33.4 | 220/494 | 44.5 | 487/1,620 | 30.1 | |
| Year of assessment | |||||||
| 2000-2005 | 657/2,114 | 31.1 | 160/494 | 32.4 | 497/1,620 | 30.7 | 0.355 |
| 2006-2010 | 689/2,114 | 32.6 | 168/494 | 34.0 | 521/1,620 | 32.2 | |
| 2011-2016 | 768/2,114 | 36.3 | 166/494 | 33.6 | 602/1,620 | 37.2 | |
| Genotype | |||||||
| 1 | 1,348/2,038 | 66.1 | 249/471 | 52.9 | 1,099/1,567 | 70.1 | < 0.001 |
| 2 | 178/2,038 | 8.7 | 47/471 | 10.0 | 131/1,567 | 8.4 | |
| 3 | 376/2,038 | 18.4 | 52/471 | 11.0 | 324/1,567 | 20.7 | |
| 4 | 111/2,038 | 5.4 | 100/471 | 21.2 | 11/1,567 | 0.7 | |
| 5/6 | 25/2,038 | 1.2 | 23/471 | 4.9 | 2/1,567 | 0.1 | |
| Race | |||||||
| White | 1,466/2,114 | 69.3 | 158/494 | 32.0 | 1,308/1,620 | 80.7 | < 0.001 |
| Black | 167/2,114 | 7.9 | 154/494 | 31.2 | 13/1,620 | 0.8 | |
| Asian | 152/2,114 | 7.2 | 147/494 | 29.8 | 5/1,620 | 0.3 | |
| Aboriginal | 68/2,114 | 3.2 | 3/494 | 0.6 | 65/1,620 | 4.0 | |
| Not stated | 261/2,114 | 12.3 | 32/494 | 6.5 | 229/1,620 | 14.1 | |
| Fibrosis stage 0-2 | 923/1,397 | 66.1 | 224/352 | 63.6 | 699/1,045 | 66.9 | 0.265 |
| Fibrosis stage 3-4 | 474/1,397 | 33.9 | 128/352 | 36.4 | 346/1,045 | 33.1 | |
| HIV Co-infection | 166/2,114 | 7.9 | 27/494 | 5.5 | 139/1,620 | 8.6 | 0.024 |
| History of alcohol use | 1,288/2,114 | 60.9 | 127/494 | 25.7 | 1,161/1,620 | 71.7 | < 0.001 |
| IDU | 1,241/2,114 | 58.7 | 100/494 | 20.2 | 1,141/1,620 | 70.4 | < 0.001 |
| Lowest income quintile | 693/2,074 | 33.4 | 139/482 | 28.8 | 554/1,592 | 34.8 | 0.015 |
ALT enzymes were higher in Canadian-born compared to immigrants (p = 0.04). Mean HCV viral load and Fi-broscan score were similar between groups. The distribution of genotype and racial background differed according to immigrant status. The rate of HIV co-infection was higher in Canadian-born (8.6%) compared to immigrants (5.5%) (p = 0.01). The prevalence of alcohol and IDU histories were greater among Canadian-born patients (p < 0.001). Approximately one-third of this sample was from the lowest quintile of neighbourhood income. Immigrants were less likely to be residents of these neighbourhoods compared to Canadian-born (p = 0.015).
Access to HCV Evaluation, Antiviral Treatment and OutcomeAccess to FibroScan and liver biopsy for the assessment of fibrosis can be used as a marker of access to HCV standard of care. In contrast to what was anticipated, the proportion of those undergoing FibroScan was greater among immigrants (42%) compared to Canadian born (32%) (p < 0.001, Figure 1A). Logistic regression analyses revealed that access to FibroScan was consistently higher among immigrants (regardless of length of time in Canada) compared to Canadian-born following adjustment for age, sex, year of assessment (Model 1), HCV risk factors and baseline laboratory data (Model 2), and race and socioeconom-ic status (Model 3). In the fully adjusted model, the odds ratio for receiving a FibroScan was 1.72 (95% CI: 1.18-2.51) among immigrants compared to Canadian born (Table 2). The proportion undergoing biopsy and/or FibroScan was greater among immigrants compared to Canadian-born in unadjusted analyses (72% vs. 66%, p = 0.008) and this effect remained after adjustment age, sex, year of first visit, and HCV risk factors (Model 2, OR 1.52, 95% CI: 1.13-2.05) (Table 2). Further adjustment for race, and socioeconomic status attenuated the strength of association (p = 0.07) although the effect remained in the fully adjusted model among recent immigrants (Model 3, OR 1.76, 95% CI: 1.05-2.97) but not long-term resident immigrants compared to Canadian-born.
Associations between immigrant status and access to diagnostic fibrosis assessment and HCV treatment.
| Fibrosis assessment and HCV treatment | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR* | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Biopsy | |||||||||
| Immigrant | 1.09 | (0.87 - 1.37) | 0.441 | 1.16 | (0.87 - 1.55) | 0.307 | 1.24 | (0.86 - 1.78) | 0.241 |
| Recent Immigrant | 1.39 | (1.02 - 1.90) | 0.037 | 1.62 | (1.09 - 2.42) | 0.016 | 2.19 | (1.31 - 3.66) | 0.003 |
| Long-Term Resident Immigrant | 0.94 | (0.69 - 1.27) | 0.670 | 0.94 | (0.67 - 1.33) | 0.724 | 1.03 | (0.69 - 1.54) | 0.884 |
| FibroScan | |||||||||
| Immigrant | 1.74 | (1.36 - 2.23) | <0.001 | 1.91 | (1.41 - 2.61) | <0.001 | 1.72 | (1.18 - 2.51) | 0.005 |
| Recent Immigrant | 1.63 | (1.16 - 2.29) | 0.005 | 1.92 | (1.25 - 2.96) | 0.003 | 1.78 | (1.05 - 3.01) | 0.032 |
| Long-Term Resident Immigrant | 1.84 | (1.33 - 2.55) | <0.001 | 1.92 | (1.33 - 2.75) | <0.001 | 1.73 | (1.14 - 2.61) | 0.009 |
| Biopsy/FibroScan | |||||||||
| Immigrant | 1.37 | (1.09 - 1.72) | 0.007 | 1.52 | (1.13 - 2.05) | 0.006 | 1.40 | (0.97 - 2.03) | 0.072 |
| Recent Immigrant | 1.42 | (1.03 - 1.95) | 0.032 | 1.72 | (1.14 - 2.61) | 0.010 | 1.76 | (1.05 - 2.97) | 0.033 |
| Long-Term Resident Immigrant | 1.33 | (0.98 - 1.81) | 0.071 | 1.33 | (0.93 - 1.88) | 0.114 | 1.21 | (0.81 - 1.81) | 0.346 |
| DAA Treatment | |||||||||
| Immigrant | 1.07 | (0.74 - 1.54) | 0.722 | 1.35 | (0.86 - 2.13) | 0.191 | 0.99 | (0.56 - 1.77) | 0.979 |
| Recent Immigrant | 0.75 | (0.44 - 1.29) | 0.304 | 0.96 | (0.50 - 1.85) | 0.910 | 0.67 | (0.29 - 1.54) | 0.348 |
| Long-Term Resident Immigrant | 1.40 | (0.88 - 2.24) | 0.157 | 1.66 | (0.97 - 2.84) | 0.063 | 1.11 | (0.59 - 2.10) | 0.741 |
| IFN ± DAA Treatment | |||||||||
| Immigrant | 0.94 | (0.65 - 1.35) | 0.722 | 0.74 | (0.47 - 1.16) | 0.191 | 1.01 | (0.57 - 1.79) | 0.979 |
| Recent Immigrant | 1.33 | (0.77 - 2.27) | 0.304 | 1.04 | (0.54 - 2.00) | 0.910 | 1.49 | (0.65 - 3.42) | 0.348 |
| Long-Term Resident Immigrant | 0.71 | (0.45 - 1.14) | 0.157 | 0.60 | (0.35 - 1.03) | 0.063 | 0.90 | (0.48 - 1.70) | 0.741 |
| Any Treatment | |||||||||
| Immigrant | 1.18 | (0.96 - 1.45) | 0.122 | 1.16 | (0.90 - 1.50) | 0.259 | 1.07 | (0.77 - 1.48) | 0.684 |
| Recent Immigrant | 1.26 | (0.94 - 1.68) | 0.118 | 1.32 | (0.92 - 1.90) | 0.137 | 1.22 | (0.77 - 1.93) | 0.388 |
| Long-Term Resident Immigrant | 1.10 | (0.83 - 1.45) | 0.519 | 1.05 | (0.77 - 1.43) | 0.754 | 0.95 | (0.67 - 1.37) | 0.798 |
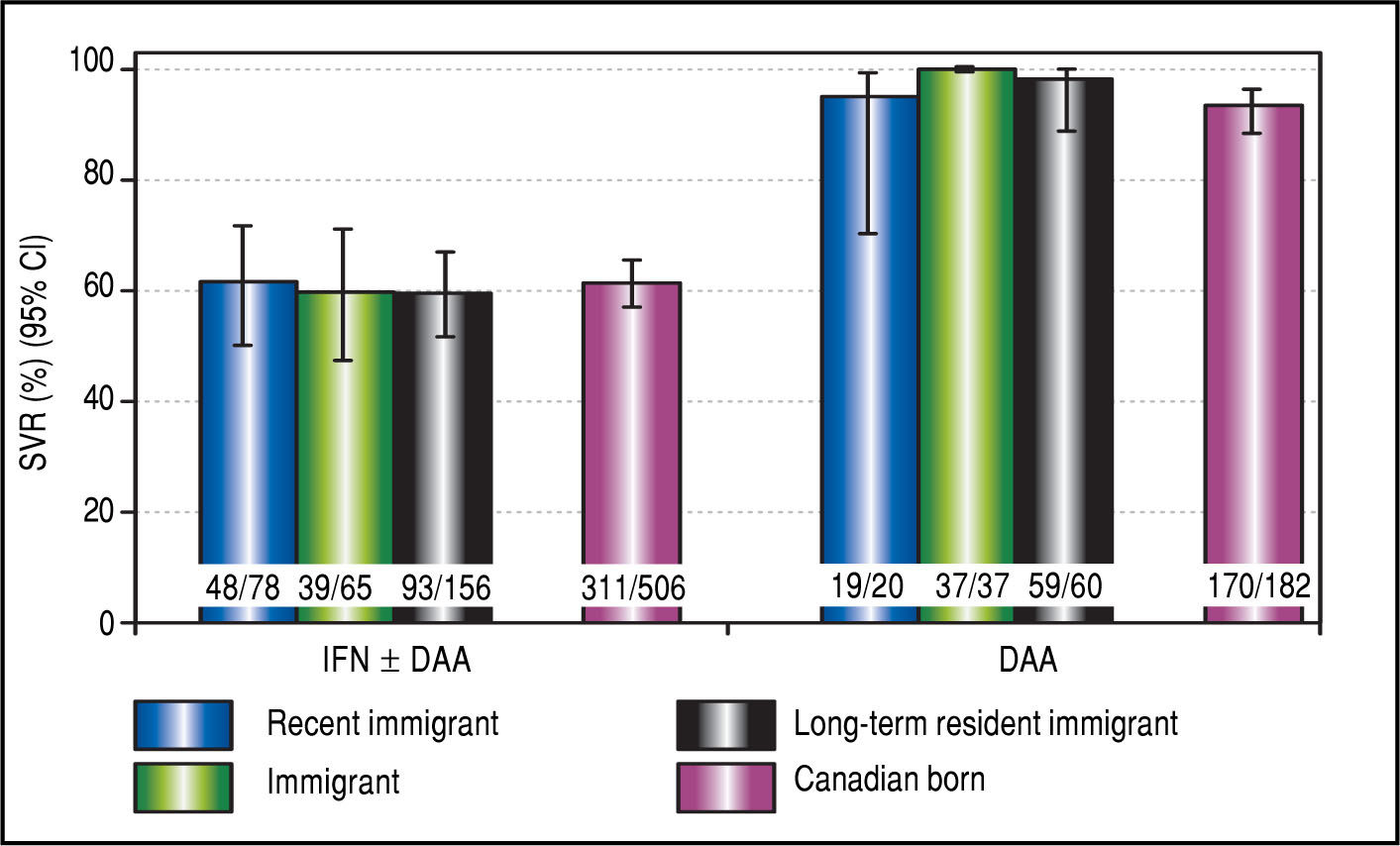
The proportion of patients initiating any HCV antiviral treatment (either IFN-based or DAA-based treatment) was similar among immigrants (49%) compared to those born in Canada (45%, p = 0.17). There were no differences in types of treatments across immigrant status; approximately one-third of immigrants and Canadians who were treated (n = 974) received DAA-based treatment compared to about 70% of patients who received pegylated in-terferon and ribavirin (± DAA, Figure 1B). Crude SVR rates (by modified intent-to-treat analyses) for IFN/PI patients were 61% overall, 59.6% in immigrants and 61.5% in Canadian born (p = 0.68). Crude SVR among DAA recipients was 94.6% overall, 98.3% among immigrants and 93.4% among Canadian born (p = 0.14, Figure 2).
Immigration status and length of time in Canada following immigration was assessed for association with SVR in logistic regression analyses with adjustment for established predictors of SVR including genotype, HCV viral load, race and HIV co-infection (Table 3). Analyses were stratified according to IFN or DAA-based therapies. Among IFN recipients, no association was identified between immigration status and likelihood of achieving SVR across three sets of models. Among DAA recipients, immigrant status was not associated with likelihood of SVR in an initial model adjusted for age, sex, and year of assessment (Model 1). The addition of HCV genotype to the model increased the odds of achieving SVR among immigrants (OR 14.1, 95% CI: 1.4-144.5, Model 2) and this was further increased with the addition of race and SES characteristics (Model 3). There were few cases of treatment failure among DAA recipients in this cohort (n = 13) which may had contributed to imprecise estimates of the effect of immigrant status on SVR as demonstrated by the large confidence intervals around the coefficient for immigrant. It was not possible to assess the effect of long-term residency among DAA recipients as no treatment failures were observed in this group.
Association between immigrant status and length of time since immigration with achieving Sustained Virologic Response for DAA and IFN-based HCV treatment.
| SVR | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR* | 95% CI | p-value | OR | 95% CI | p-value | OR | 95% CI | p-value | |
| IFN ± DAA | |||||||||
| Immigrant | 0.91 | (0.63 -1.31) | 0.598 | 0.83 | (0.56 -1.22) | 0.336 | 0.79 | (0.51 -1.20) | 0.267 |
| Recent Immigrant | 0.95 | (0.58 -1.55) | 0.830 | 0.85 | (0.51 -1.42) | 0.539 | 0.81 | (0.45 -1.43) | 0.457 |
| Long-Term Resident Immigrant | 0.95 | (0.56 -1.63) | 0.865 | 0.89 | (0.51 -1.53) | 0.671 | 0.82 | (0.47 -1.45) | 0.498 |
| DAA | |||||||||
| Immigrant | 4.58 | (0.57 -36.70) | 0.152 | 14.06 | (1.37 -144.50 | ) 0.026 | 44.24 | (2.65 -739.49) | 0.008 |
| Recent Immigrant | 1.37 | (0.16 -11.42) | 0.772 | 5.50 | (0.54 -55.92) | 0.150 | 19.46 | (1.16 -325.52) | 0.039 |
| Long-Term Resident Immigrant | - | - | - | ||||||
Using data from a large multiethnic HCV cohort, of which nearly one quarter were foreign-born, several important observations regarding the management of HCV among immigrants and non-immigrants in Canada were identified. Once engaged in HCV care, it appears that those born outside of Canada were more likely to have access to diagnostic investigations for liver fibrosis assessment with recent immigrants having more biopsies performed and long-term resident immigrants having more Fibroscans compared to those born in Canada. Initiation of HCV antiviral treatment rates (both IFN-based and DAA-based) were similar across immigration status. Immigrant patients were less likely to be from low SES areas although SES was not associated with likelihood of undergoing fibrosis assessment or HCV antiviral treatment outcomes. No differences in treatment outcomes were found by immigrant status for IFN-based therapies while the increased likelihood of achieving SVR on DAA for immigrant was likely related to sample variability given low numbers of treatment failures on all oral therapies. Unlike previous research in chronic disease,7 HCV treatment outcomes were similar between immigrants and Canadians regardless of length of time since immigration while infection management and diagnosis did not appear to converge with the Canadian-born population over time.
It is important to highlight that among those patients engaged in treatment, immigrants to Canada were more likely to have access to diagnostic and fibrosis assessment procedures compared to Canadian born patients. Our previous analyses suggested that rates of biopsy uptake were similar in this centre between immigrants and Canadian born patients.9 The explanation for this observation is unclear. It may be due to higher levels of health literacy, engagement, likelihood of retention or socioeconomic status among this group which may influence a perceived need for biopsy or Fibroscan. The current diagnostic approach in our centre is to perform Fibroscans on all patients and we would expect with time the rates to become similar between immigrants and non-immigrants. Immigrants were more likely to be better off in this cohort according to area-level income measures, which is consistent with other data from Canada which suggests that the socioeco-nomic profiles of immigrants were on par and in some cases for education exceeds that of the Canadian-born population.14,15 In our study, however, these variables were not directly associated with access to HCV assessment and we did not collect individual-level income or education data among participants.
There was a median delay of 16 years before assessment among immigrants in this cohort. Delays in accessing HCV care represent a missed opportunity to engage, treat and cure patients with HCV before progression to advanced stages of fibrosis, complications including liver failure and hepatocellular carcinoma, or develop other co-morbid illnesses which might preclude initiation of HCV antiviral therapy. Early engagement of these populations is an opportunity to prevent new incidence HCV infection post-immigration.8 In addition, about 5% of immigrants in this sample were HIV co-infected which further illustrates the importance of early infectious disease screening, preventative education and engagement in care for new immigrants. There is also a need for screening and outreach programs among lower SES groups in general regardless of immigration history.
The benefits of DAA treatments including reduced treatment duration, fewer side effects, and higher rates of adherence are associated with treatment success across all sociodemographic subgroups in our patient population. Rates of SVR exceed 90% in this population and it was not clear whether a small increase in rates of SVR among immigrants was a true effect or an artefact given the low number of treatment failures observed. Use of newer generation therapies within the multidisciplinary approach to HCV practiced in our clinic will likely engage immigrants and other socially disadvantaged groups in ways which can overcome traditional barriers to access to care, treatment, and treatment success.9,16
Although immigrants are generally found to be healthier and live longer than their Canadian-born counterparts, (the so called “healthy immigrant effect”17), they may face a variety of barriers to optimal health care and treatment which, over time, may result in the erosion of this initial health advantage over native-born populations.
This analysis was conducted in a large, representative population followed for over a decade. We utilized a well-constructed dataset with limited missing data issues. Nonetheless, several limitations are acknowledged. Retrospective analyses are often limited due to missing data. A concerted effort was made to ensure accuracy and completeness of the database. Geographic information was assigned using self-reported postal codes. This introduces a potential for misclassification if codes were incorrectly reported,18,19 although efforts were made to code individuals to their neighbourhood of residence using the PCCF program. Data on immigration status, country of origin and length of time in Canada were self-reported which may have introduced some degree of bias. Assessment of HCV genotype distribution by country of origin in this cohort was consistent with previous reports.5,20 It is clear by the cultural and regional make up of these patients that our classification has reflected the changing immigration patters to Canada over the past several decades. We did not capture language of communication in this study, although we have previously demonstrated in our clinic population that spoken language did not influence access to clinical care outcomes.9 Our analysis did not consider clinical outcomes of end stage liver disease including liver failure, hepatocellular carcinoma and liver transplantation. Given the identified delay in diagnosis and engagement in care, this should be a focus of future health outcome research in immigrants living with HCV.