Hepatitis C virus (HCV) infection is one of the leading causes of cirrhosis. As a result of chronic in-flammatory response to the virus, HCV-infected patients may be at a higher risk of venous thromboembolism (VTE). However, the data on this association is unclear. This systematic review and meta-analysis was conducted with the aims to summarize all available evidence.
Material and methodsA literature search was performed using MEDLINE and EMBASE from inception to April 2016. Studies that reported relative risks, odd ratios, or hazard ratios comparing the risk of VTE among HCV-infected patients vs. subjects without HCV infection were included. Pooled risk ratios (RR) and 95% confidence interval (CI) were calculated using a random-effect, generic inverse variance method.
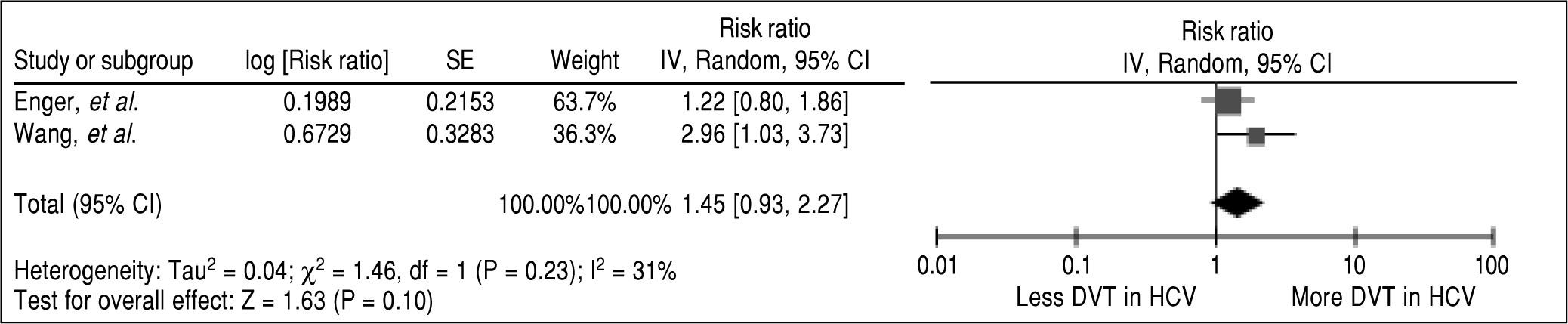
ResultsThree studies met our eligibility criteria and were included in analysis. The pooled RR of VTE in HCV-infected patients vs. subjects without HCV infection was 1.38 (95% CI, 1.08-1.77, I2 = 40%). Subgroup analysis showed that risk was increased for both pulmonary embolism (PE) and deep venous thrombosis (DVT) even though without adequate power to demonstrate statistical significance (Pooled RR of 1.34, 95% CI, 0.67-2.66 for PE and pooled RR 1.45, 95% CI, 0.93-2.77 for DVT)
ConclusionOur study demonstrated a significantly increased risk of VTE among HCV-infected patients. Further studies are required to clarify how this risk should be addressed in clinical practice.
Hepatitis C virus (HCV) infection is one of the leading causes of cirrhosis. It is estimated that approximately 180 million patients across the world are infected with this virus.1 Several extrahepatic manifestations of chronic HCV infection such as skin (porphyria cutanea tarda, vitiligo), renal (membranous glomerulonephritis) endocrine gland (diabetes, thyroiditis) and hematologic disease (cryoglobulinemia, lymphoma) have been described in about one-third of patients.2-4
Deep venous thrombosis (DVT) of the lower extremity and pulmonary embolism (PE), which are collectively known as venous thromboembolism (VTE), are major health care problems that cause up to 300,000 deaths per year in the United States.5 Several medical conditions, including cancer, surgery, immobilization, pregnancy, and chronic liver disease are well-established risk factors for VTE.6-9 Chronic inflammation has been increasingly recognized as another risk factor for VTE. In vitro studies have demonstrated provocative effects of inflammatory cytokines and oxidative stress on the coagulation cascade, resulting in hypercoagulable state.10,11 Moreover, several epidemiologic studies have shown an increased incidence of VTE among patients with chronic inflammatory conditions such as systemic lupus erythematosus, rheumatoid arthritis, psoriasis, inflammatory bowel disease and inflammatory myositis.12-17
As a result of chronic inflammatory response to the virus, HCV-infected patients may be at a higher risk of VTE. However, the data on this association is unclear.18-22 This systematic review and meta-analysis was conducted with the aims to summarize all available evidence to assess the risk of VTE among HCV-infected patients.
Material and MethodsSearch strategyTwo investigators (KW and PU) independently searched for published studies indexed in MEDLINE and EMBASE database from inception to April 2016 using the search strategy that included the terms for “hepatitis C virus” and “venous thromboembolism” described in search strategy (Table 1). No language limitation was applied. A manual search for additional relevant studies using references from retrieved articles was also performed.
Search strategy.
| Database: Ovid MEDLINE |
|---|
| 1. exp thromboembolism/ |
| 2. thromboembolism.mp. |
| 3. exp venous thrombosis/ |
| 4. venous thrombosis.mp. |
| 5. exp pulmonary embolism/ |
| 6. pulmonary embolism.mp. |
| 7. or/1-6 |
| 8. hepatitis C.mp. or exp hepatitis C/ |
| 9. exp hepatitis C virus/ |
| 10. or/8-9 |
| 11. 7 and 10 |
| Database: EMBASE |
| 1. exp thromboembolism/ |
| 2. thromboembolism.mp. |
| 3. exp venous thrombosis/ |
| 4. venous thrombosis.mp. |
| 5. exp pulmonary embolism/ |
| 6. pulmonary embolism.mp. |
| 7. or/1-6 |
| 8. hepatitis C.mp. or exp hepatitis C/ |
| 9. exp hepatitis C virus/ |
| 10. or/8-9 |
| 11. 7 and 10 |
The inclusion criteria were as follows:
- •
Case-control, cross-sectional or cohort studies published as original studies to evaluate the risk of venous thromboembolism among HCV-infected patients.
- •
Odds ratios, relative risks, hazard ratios or standardized incidence ratios with 95% confidence intervals (CI) were provided.
Study eligibility was independently determined by the two investigators noted above. Differences in the determination of study eligibility were resolved by mutual consensus. The quality of each study was also independently evaluated by each investigator using Newcastle-Ottawa quality assessment scale.23 This scale evaluated each study in three domains including the selection of the participants, the comparability between the groups and the ascertainment of the exposure for case-control study and the outcome of interest for cohort study. The modified Newcastle-Ottawa scale as described by Herzog, et al. was used for cross-sectional study.24
Data extractionA standardized data collection form was used to extract the following data from each study: title of the study, name of the first author, year of study, year of publication, country of origin, number of participants, demographic data of participants, method used to identify and verify HCV infection as well as the event of interest (venous thromboembolism), adjusted effect estimates with 95% CI and covariates that were adjusted in the multivariate analysis.
To ensure the accuracy, this data extraction process was independently performed by all investigators. Any data discrepancy was also resolved by referring back to the original articles.
Statistical analysisData analysis was performed using Review Manager 5.3 software from the Cochrane Collaboration (London, United Kingdom). Adjusted point estimates and standard errors from individual study were combined by the generic inverse variance method of DerSimonian and Laird, which assigned the weight of each study based on its variance.25 In light of the possible high between study variance, we used a random-effect model rather than a fixed-effect model. Cochran's Q test and I2 statistic were used to determine the between-study heterogeneity. A value of I2 of 0% to 25% represents insignificant heterogeneity, more than 25% but less than or equal to 50% represents low heterogeneity, more than 50% but ≤ 75% represents moderate heterogeneity, and > 75% represents high heterogeneity.26
ResultsOur search strategy yielded 3,017 potentially relevant articles (1,102 articles from Medline and 1,915 articles from EMBASE). After the exclusion of 1,064 duplicated articles, 1,953 of them underwent title and abstract review. One thousand nine hundred and twenty eight articles were excluded at this stage since they were case reports, letters, review articles or interventional studies, leaving 25 articles for a full-length article review. Sixteen of them were excluded since they did not report the outcome of interest while six articles were excluded since they were descriptive studies without comparators. Three articles, all of which were retrospective cohort studies, met the eligibility criteria and were included in the data analysis.18,20,22 Manual review of the bibliography selected studies and review articles did not yield any additional eligible studies. Figure 1 and table 2, outlines the literature review and study selection process. The clinical characteristics and the quality assessment of the included studies are described in table 2. It should be noted that the inter-rater agreement for the quality assessment using the Newcastle-Ottawa scale was high with the kappa statistics of 0.80.
Main characteristics of the studies included in this meta-analysis of the association between risk of venous thromboembolism in hepatitis C infection.
| Ahmed, et al.18 | Enger, et al.20 | Wang, et al.22 | |
|---|---|---|---|
| Country | United States | United States | Taiwan |
| Study design | Retrospective cohort study | Retrospective cohort study | Retrospective cohort study |
| Year | 2012 | 2014 | 2015 |
| Number of participants | 47,391 cases and 50,291 comparators | 22,733 cases and 68,198 comparators | 3,686 cases and 14,744 comparators |
| Cases | Patients with HCV infection were identified from a health insurance database from 2000 to 2009 | Adults (> 18 years) infected with HCV were identified from a health insurance database that covered 12 million enrollees from January 1, 2000 to September 30, 2006 | Adults (> 18 years) infected with HCV were identified from Taiwan Longitudinal Health Insurance Database that covered 1 million enrollees from 1998 - 2010 |
| Comparators | Sex and age-matched adults without HCV infection were randomly selected from the same database | Sex and age-matched adults without HCV infection were randomly selected from the same database | Sex and age-matched adults without HCV infection were randomly selected from the same database |
| Mean age of participants in years (case/comparator) | N/A | 49.0/49.0 | 51.9/51.4 |
| Percentage of female (case/comparator) | 39.0 / 39.0 | 37.6/37.6 | 48.9 / 48.9 |
| Identification and verification of hepatitis C infection | At least 2 diagnostic codes of HCV infection within 6 months of each other from the database | Diagnostic codes of HCV infection from the database | Diagnostic codes of HCV infection from the database |
| Definition of VTE | DVTandPE | DVTandPE | DVTandPE |
| Identification and verification of VTE | Diagnostic codes of DVT and PE from the database plus at least on prescription of anticoagulant within 90 days of the VTE diagnosis | Diagnostic codes of DVT and PE from the database | Diagnostic codes of DVT and PE from the database |
| Confounder adjustment | None | Age, sex, hypertension, Steroid use, recent use of interferon treatment | Age, sex, comorbidities of DM, HTN, hyperlipidemia, CVA, heart failure, lower leg fracture or surgery, Cancer, pregnancy, alcohol-related illness, COPD, CRF, nephrotic Syndrome, IBD, SLE, and RA, and medications of estrogen, selective estrogen receptor modulator, drugs used in fertility control, low-molecular weight heparin and warfarin |
| Quality assessment (Newcastle-Ottawa scale) | Selection: 3 Comparability: 2 Outcome: 3 | Selection: 3 Comparability: 2 Outcome: 3 | Selection: 4 Comparability: 2 Outcome: 3 |
In the overall analysis, we found that HCV-infected patients had a significantly higher risk of VTE compared with subjects without HCV infection with the pooled risk ratio (RR) of 1.38 (95% CI, 1.08-1.77, P = 0.009). The statistical heterogeneity was low with an I2 of 40%. Two studies provided data on each subtype of VTE (i.e., PE and DVT).20,22 Therefore, we also performed a pooled analysis of the subtypes. We found a higher risk of PE and DVT among HCV-infected patients with the pooled RR 1.34 and 1.45 even though without a statistical significance (95% CI of 0.67-2.66, P = 0.40 and 0.93-2.26, P = 0.10, respectively). The forest plots of VTE, PE and DVT analysis are shown in figures 2, 3 and 4, respectively.
We did not perform the evaluation for publication bias as the number of studies included in the meta-analysis was too small.
DiscussionThis study is a systematic review and meta-analysis assessing the risk of VTE among HCV-infected patients. We found that the risk of VTE were significantly higher among HCV-infected patients compared with subjects without HCV infection. The increased risk was found in both subtypes of VTE (PE and DVT) even though the result of the subgroup analysis did not reach statistical significance as only two studies were included.
Interestingly, there was a recent meta-analysis of the risk of VTE in HCV patients conducted by Ambrosino, et al.27 Our methodology is different. In that study, the authors included both studies that were conducted in general population and studies that were conducted in specific group of population (that is; perioperative patients in Best, et al. study,19 intravenous drug users in RostamiJalilian, et al. study,28 and cirrhotic patients in El Bokl, et al. study29).
Since baseline VTE risk among these specific groups19,28,29 is not similar to general population, therefore, we did not combine them with studies from general population and did not include them in our meta-analysis. In fact, the meta-analysis by Ambrosino, et al.27 had high between-study variation (I2 of 91% in the main analysis) which suggests that the primary studies were too heterogeneous to combine. On the other hand, this study has I2 of only 40% which suggests that the methodological approach is more suitable.
The precise mechanisms behind the association of VTE and HCV infection are not known. There are few possible explanations.
The first explanation is related to chronic inflammation. As previously mentioned, increasing amount of evidence have linked chronic inflammation to hypercoagulable state. Chronic inflammation appears to increase the tendency of clotting through the stimulation the coagulation cascade by inflammatory cytokines.11,30,31 Furthermore, initiation of blood clots may happen more frequently among HCV-infected patients as a result of endothelial dysfunction due to oxidative stress related to inflammation.32,33
Second, chronic HCV infection is associated with abnormal autoantibody production. A recent study has demonstrated that up to 6% of HCV-infected patients had antiphospholipid antibody, an autoantibody that is strongly linked to hypercoagulability.34 Chronic HCV infection is also associated with excessive production of cryoglobulin. High level of cryoglobulin is associated with serum hyperviscosity which might serve as another predisposing factor to thrombosis.35
Although all of the included studies were of high quality as reflected by the high quality assessment scores, we acknowledged that this meta-analysis had some limitations and the results should be interpreted with caution.
First, the primary studies included in this meta-analysis did not adjust for several known risk factors for VTE including smoking, body mass index, and hospitalization as their databases did not have such information. Thus, it is possible that the observed association is a result of confounding. Second, we could not perform evaluation for publication bias as the number of included studies was too small. Thus, publication bias in favor of positive studies might have been present. Third, we cannot exclude the possibility of surveillance bias as patients with chronic disease, including chronic HCV infection, might undergo more medical examinations and investigations, leading to a higher likelihood of VTE detection.
In summary, this meta-analysis demonstrated an increased risk of VTE among HCV-infected patients. Further studies are required to clarify how this risk should be addressed in the clinical practice.
Abbreviations- •
COPD: chronic obstructive pulmonary disease.
- •
CRF: chronic renal failure.
- •
CVA: cerebrovascular accident.
- •
DM: diabetes mellitus.
- •
DVT: deep vein thrombosis.
- •
HCV: hepatitis C virus.
- •
HTN: hypertension.
- •
IBD: inflammatory bowel disease.
- •
ICD-9-CM: International Classification of Disease.
- •
Revision 9: clinical modification.
- •
IRR: incidence rate ration.
- •
LHID: Longitudinal Health Insurance Database.
- •
PE: pulmonary embolism.
- •
PVT: portal vein thrombosis.
- •
RA: rheumatoid arthritis.
- •
SLE: systemic lupus erythematosus.
- •
VTE: venous thromboembolism.
The authors have no commercial associations that might be a conflict of interest in relation to this article.
FundingNone.
FundingNone.
Conflict of Interest Statement for all AuthorsWe do not have any financial or non-financial potential conflicts of interest.
Authors’ ContributionsAll authors had access to the data and a role in writing the manuscript.