Hypoxia-inducible factor-1α is critically involved in the pathogenesis of liver diseases. Its inhibitor genistein attenuated D-galactosamine (D-GalN)-induced liver damage. However, the role of genistein in acute-on-chronic liver failure (ACLF) is unclear. The influence of genistein on reactive oxygen species (ROS) and hepatocyte functions were evaluated in a rat model of ACLF.
Material and methodsGenistein [20mg/ (kg. day)]/coenzyme Q10 [10mg/ (kg. day)]/lipoic acid [20mg/ (kg. day)] was administered via the intra-gastric route daily for 6 weeks as co-treatment to the rats in the experimental groups. Then, 100μg/kg LPS combined with 0.5g/kg D-GalN was injected intraperitoneally to attack the rats.
ResultsGenistein significantly attenuated LPS/D-GalN-induced ACLF, characterized by ameliorated gross appearance and microscopic histopathology of liver, reduced AST level in serum, whereas increased levels of ATP, ADP/O, and respiratory control ratio (RCR) in mitochondria. Genistein suppressed necrosis and ROS production.
ConclusionThese results suggested that genistein could protect against ACLF through inhibiting cellular ROS production and necrosis, improving RCR, and decreasing permeability transition pores in mitochondrial, which was similar as mitochondrial protective agent coenzyme Q10.
Acute-on-chronic liver failure (ACLF) is acute deterioration of established chronic liver diseases, with a high mortality from 30% to 70%.[1] ACLF has extremely poor prognosis, as a major indication for liver transplantation. However, the pathogenesis of ACLF remains poorly understood. Hypoxia-inducible factor (HIF) comprises an oxygen-sensitive α-subunit and a constitutively expressed β-subunit.[2] HIF-α consists mainly of HIF-1α and HIF-2α subunits.[3] HIF-1α is a transcription factor regulating oxygen homeostasis, which orchestrates a wide range of genes involved in oxygen tension, glycolysis, angiogenesis, cell death, and so forth.[4] HIF-1α is crucial in regulating oxygen tension. Under hypoxia, HIF-1α is translocated to the nucleus and binds to β-subunit, leading to transcription of target genes[5].HIF-1α contributes to early hepatocellular necrosis in acetaminophen toxicity–induced ACLF .[6]
Genistein is one of the major isoflavones in unprocessed soy .[7] It is abundant in a variety of soy products consumed worldwide. Previous studies reported that genistein blocked the apoptotic and anti-necrotic pathways by modulating the expression of pro-apoptotic and anti-necrotic genes.[8] Genistein, as an inhibitor of HIF-1α, exhibited strong anti-angiogenic activity by inhibiting the activities of tyrosine kinase.[9] Genistein exerted a protective effect on diabetic nephropathy through regulating oxidative stress, especially for diabetic patients with moderate blood glucose levels [10]. Genistein served as a chemotherapeutic agent in different types of cancers, mainly by inhibiting metastasis and regulating apoptosis.[11] Genistein also attenuated D-galactosamine (D-GalN)-induced liver damage and fibrosis.[12] Long-term chronic liver injury results in kidney injury, cirrhosis and hepatocellular cancer. Advanced chronic liver diseases pathologically affect kidney perfusion and circulation. Patients with chronic liver diseases were susceptible to acute kidney injury.[13] Chronic liver diseases significantly increase the risk of hepatocellular carcinoma. Molecular mechanisms underlying hepatocellular carcinoma include inflammation, chronic tissue injury, epigenetic deregulation, genome instability, and so on.[14] This study aimed to investigate the potential mechanisms underlying hepatoprotective effect of genistein on ACLF.
2MATERIAL AND METHODS2.1Animals and treatmentsEight-week-old male Sprague–Dawley (SD) rats (weighing approximately 200g) were purchased from the Academy of Military Medical Sciences Beijing, China. Food and water were provided ad libitum. The rats were maintained on a 12-h light–dark cycle in an environment with humidity of 55±5% and temperature at 18–21°C for 1 week before the experiments. SD rats were randomly divided into five groups. The rats were housed in clear cages with 10 animals per cage.
2.2Ethical approvalAll experiments were approved by the ethics committee of the You’ an Hospital, an affiliated hospital of Capital Medical University, Beijing, China. Animal care was performed according to the applicable health guidelines.
2.3Induction of acute-on-chronic liver failure and genistein/coenzyme Q10/lipoic acid co-treatment.The animals were randomly divided into six groups (n=10/group). The grouping and treatment regimen were as follows: Group I (normal control): The rats received vegetable oil (1.5mL/kg), once every 3 days for 6 weeks via intraperitoneal injection.
Group II (genistein control): The rats received genistein [20mg/ (kg. day)] for 6 weeks.
Group III (model group): The rats received 50% carbon tetrachloride vegetable oil solution (1.5mL/kg), once every 3 days for 4 weeks via intraperitoneal injection, and then the dose was increased to 2mL/kg for 2 weeks.
Group IV (genistein co-treatment): The rats received 50% carbon tetrachloride vegetable oil solution (1.5mL/kg) and genistein [20mg/ (kg. day)] for 6 weeks.
Group V (lipoic acid co-treatment): The rats received 50% carbon tetrachloride vegetable oil solution and lipoic acid [20mg/ (kg. day)] for 6 weeks.
Group VI [coenzyme Q10 (CoQ10) co-treatment]. The rats received 50% carbon tetrachloride vegetable oil solution and COQ10 [10mg/ (kg. day)] for 6 weeks.
On the basis of chronic liver injury, the rats in Groups II to VI were administered lipopolysaccharide (LPS) (100μg/kg) combined with D-galactosamine (D-GalN) (0.5g/kg) intraperitoneally to induce acute liver failure. After 96h, the animals were sacrificed by cervical dislocation.
2.4Mitochondrial isolation.Rat hepatocytes were homogenized at a high speed in homogenization buffer(containing 100mM KCl, 25mM Tris-HCl, pH 7.5, protease inhibitor cocktail, and 0.4M sucrose) for 20s at 4C. The homogenate was centrifuged at 900g for 10min at 4°C.
The supernatant was then centrifuged at 14,000g for 15min and washed with homogenization buffer once. The mitochondrial pellet was preserved at−80°C. The protein concentration of the mitochondrial pellet was measured.
2.5Western blot analysisProteins were extracted from the liver tissues. 50μg proteins was subjected to12% sodium dodecyl sulfate–polyacrylamide electrophoresis gel and transferred on to polyvinylidene difluoride membranes. The membranes were incubated with primary antibodies against HIF-1α, caspase 1, caspase 8 and β-actin (1:1000; Sigma, St. Louis, USA) overnight at 4°C. The membranes were washed with Tris-buffered saline with Tween 20. Then, secondary antibody (1:10000; Santa Cruz, CA USA) was added using the established techniques.[15] The antigen–antibody complexes were visualized with enhanced chemiluminescence (ECL) (ECL-Kit, Amersham, USA) according to the manufacturer's instruction.
2.6Mitochondrial respiratory control ratio.Mitochondrial fractions of liver tissues were incubated with GENMED reagent A at 25°C. Reagent B (state 4 substrate solution) and reagent C (state 3 substrate solution) were added in a proper sequence. The ratio of respiration rate of state 3 to state 4 in mitochondrial fractions represented the mitochondrial respiratory control ratio (RCR). The kit from GENMED Scientifics Inc, Beijing, China, was used for measurement.
2.7Measurement of intracellularATPATP contents within mitochondrial fractions were measured using the ATP bioluminescent assay kit (GENMED). Mitochondrial lysates were collected, and luminescence was quantified to detect the ATP content within mitochondrial fractions according to the manufacturer's instruction.
2.8Detection of mitochondrial permeability transition pore (MPTP) openingThe MPTP fluorescence detection kit (GENMED Scientifics Inc, Shanghai, China was used to measure MPTP opening. Calcein was used to stain mitochondria. This dye was selectively aggregated inside the mitochondria, as demonstrated by green fluorescence. When MPTP opened, the dye was released from the mitochondria. Changes in mitochondrial fluorescence represented the degree of MPTP opening. The sections were observed under a laser scanning confocal microscope (LSM 510META; Zeiss, Germany). The fluorescence intensity of mitochondria was measured using fluorescence spectrophotometry (Victor X; PerkinElmer, Hamburg, Germany).
2.9Reactive oxygen species stainingLiver tissues (5 mm3 in volume) were cut into pieces to obtain single cells using the single-cell suspension preparation equipment. The nylon membrane (200-mesh) was used to filter the single-cell suspension. The suspension was centrifuged at 4000g for 10min at 4°C. The single-cell precipitate was resuspended, and the supernatant was discarded. Reactive oxygen species (ROS) fluorescent probe 2’, 7’-dichlorofluorescin diacetate (DCFH-DA, Sigma, St. Louis, USA) was added to the precipitate and incubated for 30min in a 37°C incubator. Stained single-cell suspension was centrifuged at 12,000g for 8min. The precipitate was washed with phosphate-buffered saline (PBS) precipitate. The fluorescence intensity of single cell suspension was measured at random using flow cytometry.
2.10Electron microscopyGlutaraldehyde (3%) in 0.2M sodium cacodyls ate was used to fix liver tissue samples. The samples were washed three times with PBS. After dehydration using serial concentrations of ethanol, the cells were embedded in Epon. The images were acquired with a transmission electron microscope.
3Liver function testThe serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured with an AU400 automatic biochemical analyzer.
3.1Statistical analysisData were analyzed using SPSS (SPSS, IL, USA) and Graph Pad Prism version 5 software. Statistical differences were assessed by performing one-way analysis of variance followed by the Student t test. All data represent at least three independent experiments. Differences were considered statistically significant at confidence levels of P<0.05 (*) or P<0.01 (**).
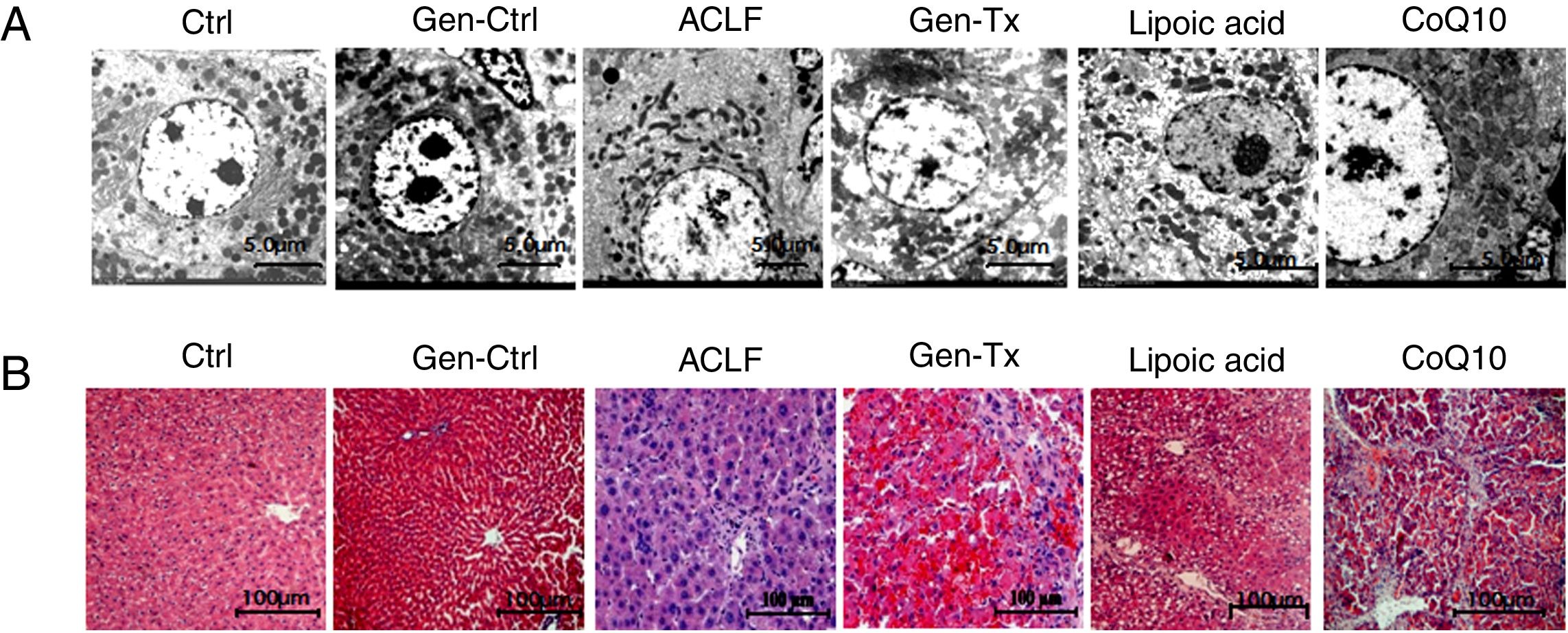
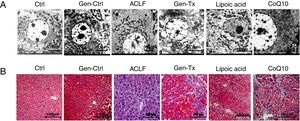
4RESULTS4.1Establishment of ACLF rat modelThe SD rat model of ACLF was established as previously described [16]. The pathological changes in liver tissues were evaluated with hematoxylin and eosin staining (HE). The livers were rosy and smooth with intact lobule structure in the control group (Group I) and genistein control group (Group II). However, the livers of rats in the model group (Group III) became small and hard accompanied by necrosis; with small nodules on their surface (Figs. 1A and B).
Genistein ameliorates gross and microscopic liver histopathology. Rats were treated with vegetable oil/genistein alone for 6 weeks as the control group(GroupI and Groups II);50% carbon tetrachloride vegetable oil solution for 6 weeks as the model group(Groups III);50% carbon tetrachloride vegetable oil solution and genistein/ lipoic acid / coenzyme Q10 for 6 weeks respectively as the co-treatment group(Group IV, Group V, and Group VI).The rats in groups III to VI were administered LPS(100μg/kg) combined with D-galactosamine (D-GalN) (0.5g/kg) intraperitoneally to induce acute liver failure.(A-B)Gross liver histopathology (100×) in GroupI, II, III, IV,V and VI.(C) Electron micrographs of hepatocyte in GroupI, II, III, IV, V and VI.(D) Hematoxyl & eosin staining in GroupI, II, III, IV, V and VI.
Electron microscopy was used as the gold standard for ultrastructure. The nuclear heterochromatin was intact in Group I and Group II. The nuclear chromatin was fragmented, and the rough endoplasmic reticulum was invisible in the model group (Fig. 1C). Thus, the ACLF model was established successfully.
4.2Genistein ameliorated gross and microscopic liver histopathology.Genistein exerted anti-inflammatory effects since the aglycone of genistein is HIF-1α inhibitor.[17] The liver became softer and smooth in the genistein co-treatment group (Group IV) compared with Group III (Figs. 1A and 1B). The HE-stained liver sections from the genistein co-treatment group (Group IV) exhibited comparatively normal hepatocytes with intact nucleus and complete lobular structures, although a few hepatocytes displayed macrovesicular steatosis and mild swelling (Fig. 1D). Electron microscopy indicated no nuclear fragmentation or dissolution in Group IV compared with Group III (Fig. 1C).
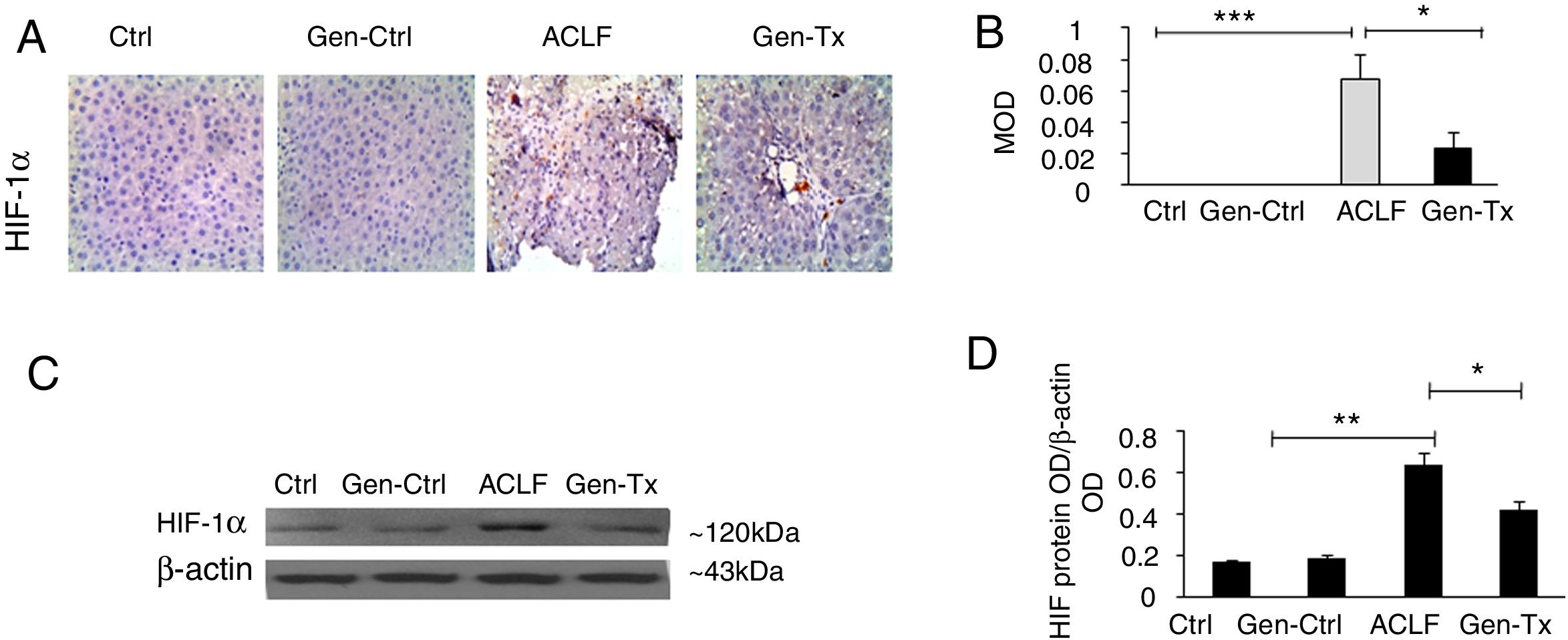
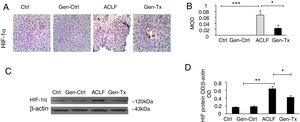
4.3Expression of HIF-1α in the mice of the model group (Group III) and Genistein co-treatment group (Group IV).Hypoxia was implicated in the pathogenesis of liver diseases. Under hypoxic conditions, HIF stimulated homeostatic response to liver fibrosis, cirrhosis and hepatocellular carcinoma. The expression level of HIF-1α was measured in Groups III and IV using immunohistochemistry (IHC) and Western blot to explore genistein, the inhibitor of HIF-1α, as interventional approach for ACLF. As revealed by IHC, the expression of HIF-1α protein was remarkably increased in Group III than in Group I and Group II. The expression of HIF-1α was decreased significantly in Group IV than in Group III (Figs. 2A and 2B). The results from Western blot were consistent with the findings from IHC (Figs. 2C and 2D). Overall, genistein co-treatment protected the hepatocytes from histopathological changes during the development of ACLF.
Expression of HIF-1α and the effect of its inhibitor genistein on serum biochemical parameters. Different correspondence letters indicate significant differences at P<0.05 by the Student t test.* p <0.05, ** p <0.001 (A, B) immunohistochemistry exhibited the expression of HIF-1α in Group-I, II, III and IV. (C, D) Western blot exhibited the expression of HIF-1αin Group-I, II, III and IV.
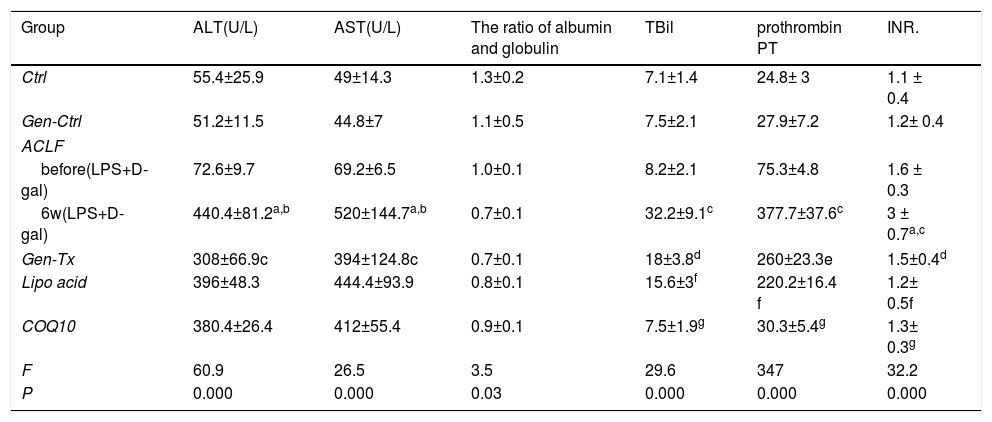
Serum levels of AST and ALT, as well as the ratio of albumin to globulin are markers of hepatocyte necrosis. These parameters were detected to assess the damage of hepatocytes (Table 1). Serum levels of ALT and AST were increased significantly in the model group (Group III) compared with the control group (Group I) or genistein co-treatment group (Group II). The levels of these enzymes were decreased in genistein co-treatment group (Group IV) compared with model group (Group III) significantly. Thus, genistein exerted a protective effect on liver necrosis in ACLF.
Effect of genistein on serum biochemical parameters.
| Group | ALT(U/L) | AST(U/L) | The ratio of albumin and globulin | TBil | prothrombin PT | INR. |
|---|---|---|---|---|---|---|
| Ctrl | 55.4±25.9 | 49±14.3 | 1.3±0.2 | 7.1±1.4 | 24.8± 3 | 1.1 ± 0.4 |
| Gen-Ctrl | 51.2±11.5 | 44.8±7 | 1.1±0.5 | 7.5±2.1 | 27.9±7.2 | 1.2± 0.4 |
| ACLF | ||||||
| before(LPS+D-gal) | 72.6±9.7 | 69.2±6.5 | 1.0±0.1 | 8.2±2.1 | 75.3±4.8 | 1.6 ± 0.3 |
| 6w(LPS+D-gal) | 440.4±81.2a,b | 520±144.7a,b | 0.7±0.1 | 32.2±9.1c | 377.7±37.6c | 3 ± 0.7a,c |
| Gen-Tx | 308±66.9c | 394±124.8c | 0.7±0.1 | 18±3.8d | 260±23.3e | 1.5±0.4d |
| Lipo acid | 396±48.3 | 444.4±93.9 | 0.8±0.1 | 15.6±3f | 220.2±16.4 f | 1.2± 0.5f |
| COQ10 | 380.4±26.4 | 412±55.4 | 0.9±0.1 | 7.5±1.9g | 30.3±5.4g | 1.3± 0.3g |
| F | 60.9 | 26.5 | 3.5 | 29.6 | 347 | 32.2 |
| P | 0.000 | 0.000 | 0.03 | 0.000 | 0.000 | 0.000 |
Ctrl: normal control; Gen-Ctrl: Genistein-control ; ACLF :acute-on-chronic liver failure : Gen-Tx: genistein co-treatment; Lipoic acid :lipoic acid co-treatment; COQ10: coenzyme Q10 co-treatment; ALT: Alanine aminotransferase ; AST :aspartate aminotransferase;Note:
Note: a P<0.01 Ctrl vs. ACLF(6w); b P<0.01 Gen-Ctrl vs ACLF(6w). ; c P<0.01 ACLF(before) vs ACLF(6w); d P<0.05 Gen-Tx vs. ACLF(6w); e P<0.01 Gen-Tx vs. ACLF(6w); f P<0.01 Lipoic acid vs ACLF(6w).; g P<0.01 COQ10 vs ACLF(6w).
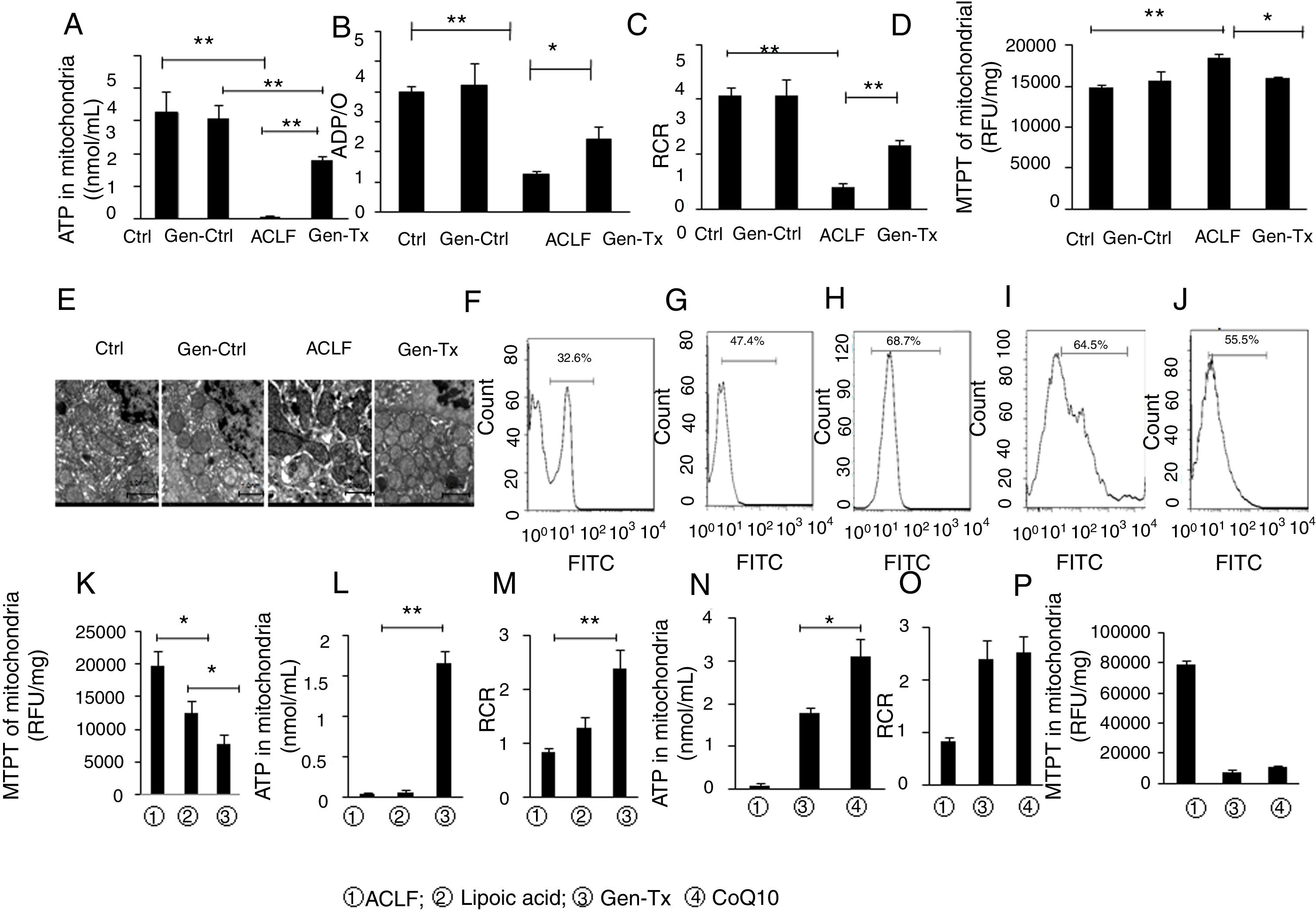
The oxygen delivered to cells is consumed mainly by the mitochondria. The ATP level in the mitochondria reflects the functional status of mitochondria. ATP content, ADP/O, and RCR were detected to explore whether genistein co-treatment could protect liver from failure by attenuating the mitochondrial injury.
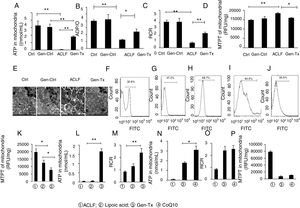
ATP content in the mitochondria decreased significantly in the model group (Group III) compared with the control group (Group I) or genistein co-treatment group (Group II). In contrast, genistein co-treatment increased ATP content significantly compared with Group III (Fig. 3A). Oxyphosphorus ratio is the ratio of oxygen consumption to inorganic phosphorus during breathing. It represents the number of ATP produced by a pair of electrons transmitted to molecular oxygen via the respiratory chain. The ADP/O was consistent with ATP content (Fig. 3B). The level of RCR confirmed the results of ATP and ADP/O (Fig. 3C).
Effects of genistein on mitochondrial function and ROS production.Mitochondrialwas isolated from rat hepatocytes. Liver tissues were cut into pieces to obtain single cells using the single-cell suspension preparation equipment The suspension was centrifuged at 4000g for 10min at 4°C. The single-cell precipitate was resuspended. ROS fluorescent probe 2’, 7’-dichlorofluorescin diacetate was added and fluorescence intensity of single cell suspension was measured at random using flow cytometry. Different correspondence letters indicate significant differences at P<0.05 by the Student t test.* p <0.05, ** p <0.001. ATP content in mitochondriain GroupI, II, III and IV. (B) ADP/O level in GroupI, II, III and IV. (C)Mitochondrial respiratory control ratio (RCR) in GroupI, II, III and IV. (D) The detection of MPTP opening in GroupI, II, III and IV.(E) The ultrastructure of mitochondria in GroupI, II, III and IV. (F-J)ROS level in GroupI, II, III, IV and V (K-M) MTPT, ATP content and RCR of mitochondrial in Group III, IV and V.(N-P) ATP content, RCR and MTPT of mitochondrial in, Group III, IV and VI.
MPTP is a nonselective mitochondrial channel. The opening of MPTP results in the disorder of mitochondrial membrane potential and reduced ATP content [18]. The level of MPTP decreased significantly in the genistein co-treatment group (Group IV) compared with the model group (Group III) (Fig. 3D).
The ultrastructure and function of mitochondria were further analyzed. Electron microscopy showed that the mitochondrial membrane was intact with clear cristae in the control group (Group I) or genistein co-treatment group (Group II) (Fig. 3E). In contrast, nuclear fragmentation and dramatic changes in mitochondrial morphology characterized by disappearance of cristae but formation of numerous vacuoles was observed in the model group (Group III) (Fig. 3E). The cell membrane integrity and the clarity of cristae in mitochondria were better in the genistein co-treatment group (Group IV) than in the model group (Group III).
4.6Genistein protected against mitochondrial injury in mice with ACLF via regulation of ROS production partly in ACLF.Mitochondrial respiratory dysfunction was crucial in multiple-organ failure induced by sepsis. Due to the production of ROS, multiple-organ failure may develop with elevating tissue oxygenation.[19] A previous study reported that mitochondrial dysfunction occurred in response to ROS generation in acetaminophen-induced hepatotoxicity in mice.[20] Therefore, the effect of genistein on intracellular ROS production was examined in LPS/D-gal-induced ACLF.
Genistein co-treatment suppressed the cellular ROS level compared with the model group (Group III) (Figs. 3F–3H). However, whether genistein protected against LPS/D-gal-induced hepatocyte injury by suppressing the intracellular burden of ROS formation was still unclear. Therefore, the effect of ROS clearance rate on mitochondrial protection was assessed.
ROS inhibitor lipoic acid was used to explore the effect. In the lipoic acid co-treatment group (Group V), the liver became hard (Figs. 1A, 1B) and HE-stained liver sections exhibited hepatic fibrosis and hepatocellular necrosis (Fig. 1D). Electron microscopy identified intact nuclear heterochromatin and scattered deformation of mitochondria in Group V (Fig. 1C). And the ROS level was lower in the lipoic acid co-treatment group (Group V) than in the genistein co-treatment group (Group IV) (Figs. 3I and 3J). Both lipoic acid and genistein co-treatment decreased the hepatocyte MTPT level significantly compared with the model group (Fig. 3K). But genistein co-treatment significantly increased the ATP and RCR levels in mitochondria compared with the model group, while lipoic acid co-treatment could not (Figs. 3L and 3M). These data suggested that genistein protected against mitochondrial injury in ACLF partly via regulation of ROS production.
4.7Comparison of mitochondrial protection between genistein and mitochondrial antioxidant coenzyme Q10.CoQ10, as a mitochondrial antioxidant, has been linked to clinical mitochondrial diseases.[21] We further compared the protection of mitochondria by genistein and coenzyme Q. The livers of rats in the COQ10 group (Group VI) became small and hard (Figs. 1A and 1B). HE-stained liver sections exhibited hepatic cirrhosis and hepatocellular necrosis (Fig. 1D). Electron microscopy identified fragmented nuclear chromatin in Group VI (Fig. 1C).
It was reported CoQ10 deficiency could disturb oxidative stress and mitochondrial bioenergetics, as demonstrated by increased ROS production and decreased ATP generation.[22] ATP, MTPT, and RCR levels in mitochondria were examined in the coenzyme Q co-treatment group (Group VI) and genistein co-treatment group (Group IV) to explore the extent of mitochondrial protection exerted by genistein. ATP levels were increased in the CoQ10 co-treatment group (Group VI) compared with the genistein co-treatment group (Group IV) (Fig. 3N), however, no difference in RCR and MTPT levels were observed between the two groups, indicating that genistein co-treatment and coenzyme Q10 co-treatment had a similar effect on improving the mitochondrial RCR and decreasing the MTPT level (Figs. 3O and 3P).
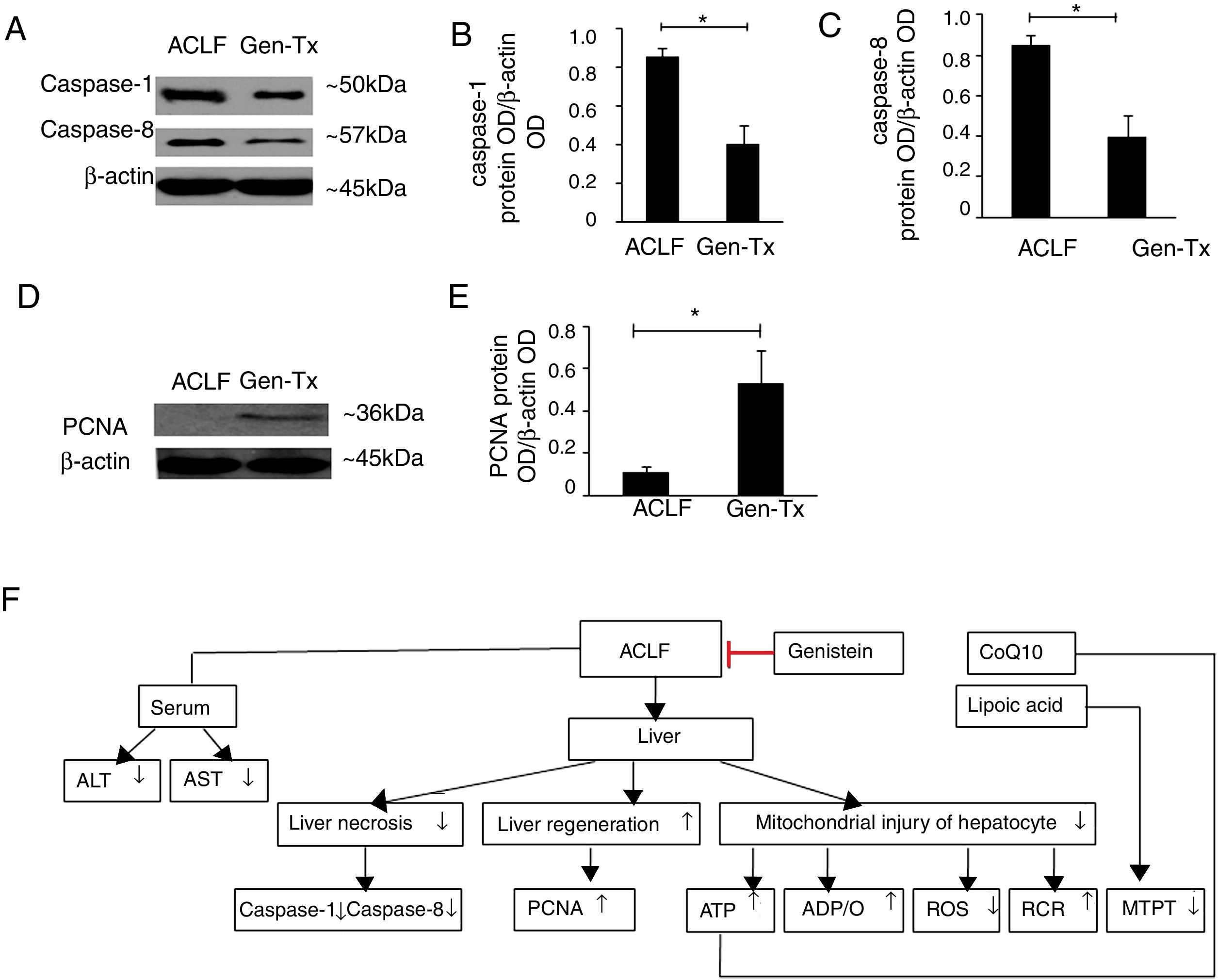
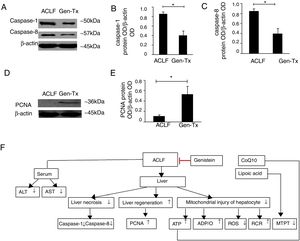
4.8Effect of genistein on necrosis and liver regeneration in rat livers.LPS could mediate inflammatory responses in ACLF.[23] Genistein alleviated hepatic damage through anti-inflammatory effects on alcohol-induced liver injury [24].Therefore, we hypothesized that genistein might decrease necrosis of hepatocytes in ACLF. The levels of caspase-1 and caspase-8 were decreased significantly in the genistein co-treatment group (Group IV) compared with the model group (Group III) (Figs. 4A-4C). We also examined the expression of proliferating cellular nuclear antigen (PCNA). The expression of PCNA in genistein co-treatment group (Group IV) was increased significantly compared with the model group (Group III) (Figs. 4D-4E); indicating the regeneration rate was higher in Group IV than Group III. Taken together, these data suggested that genistein co-treatment might decrease necrosis and promote liver regeneration (Fig. 4F).
Effect of genistein on necrosis and liver regeneration. Different correspondence letters indicate significant differences at P<0.05 by the Student t test.* p <0.05, (A-C) the expression of caspase1 and caspase8. (D, E) the expression of PCNA. (F) Scheme of postulated regulatory pathways for the effects of genistein on mitochondrial. Liver necrosis and regeneration in acute-on-chronic liver failure.
ACLF, acute-on-chronic liver failure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ATP, adenosine-triphosphate; ADP, adenosine-diphosphate; RCR, respiratory control ratio; ROS, reactive oxygen
species; MTPT, mitochondrial permeability transition pore; PCNA, proliferating cellular nuclear antigen.
ACLF is defined as acute deterioration of liver function based on pre-existing chronic liver disease.[25] It is characterized by rapid progression and high mortality of 50%–90%. In some cases, ACLF exhibits a poor prognosis and requires liver transplant.[26] At present, pharmacologic approaches to prevent mortality from ACLF are very limited. HIFs were involved in chronic liver diseases. Therefore, genistein, as an inhibitor of HIF-1α, became a hotspot for ACLF research. Genistein exerted a hepatoprotective effect by blocking the necrosis pathway in rats with fulminant hepatic failure. Genistein also attenuated D-GalN-induced liver fibrosis and liver damage. However, the role of genistein in ACLF remains unclear. The present study has demonstrated a protective effect of genistein against ACLF induced by carbon tetrachloride vegetable oil solution /LPS/D-GalN in rats.
In liver diseases, oxidative stress was induced via several mechanisms: decreased antioxidant synthesis, enhanced generation of ROS, increased release of oxidant enzymes, and impaired neutrophil function. The ROS were generated when the balance between production and utilization of free radicals was disturbed. The ROS led to cellular dysfunction, including alteration in cell membrane permeability mediated by lipid oxidation.[27] This is consistent with our data that both ROS inhibitor lipoic acid and antioxidant genistein could ameliorate mitochondrial MTPT via ROS scavenging. We found ROS levels were increased in ACLF, which is in line with a previous study.[28] They reported cerebral oxidative stress (e.g., ROS) could be induced by high ammonia concentrations in acute liver failure, and thus contributing to hepatic encephalopathy. Hepatic encephalopathy is a serious complication of ACLF. Therefore, hepatic encephalopathy caused by ROS is indirect evidence of the elevating ROS levels in ACLF.
The present study indicated that genistein protected against mitochondrial injury in ACLF partly via blocking ROS production. These data were consistent with the findings of a previous study (Sang-Hyunetal et al.), which proved that genistein inhibited osteoclast differentiation stimulated by receptor activator of nuclear factor-κB ligand. Genistein might control ROS generation through suppressing the disruption of mitochondrial electron transport chain system and ROS scavenging.[29]
We found that ROS inhibitor lipoic acid preserved mitochondrial function by ameliorating MTPT but not increasing ATP and RCR levels. Interestingly, genistein significantly increased ATP and RCR levels, as well as decreased MTPT compared with lipoic acid, implying that genistein not only attenuated mitochondrial damage by lowering ROS levels, but also protected mitochondria through other signaling pathways. Ajaz et al. reported that genistein protected against fibrosis associated with chronic liver disease via inhibiting TGF-β/Smad signaling pathway. ACLF results from pre-existing chronic liver diseases could develop to long-standing cirrhosis prior to acute deterioration of liver function. Yuan et al. reported that genistein could modulate the antioxidant enzyme levels and metabolic activities to prevent acetaminophen (APAP) -induced liver toxicity mediated by resistance to oxidative stress.[30] Therefore, it was hypothesized that genistein attenuated mitochondrial damage partly through ROS scavenging and other signal transduction pathways, such as inhibiting TGF-b/Smad signaling.
CoQ10 is a lipid-soluble component in the mitochondrial inner membrane. As an electron carrier, CoQ10 participates in ATP production from complexes I and II to complex III, as an important part of the mitochondrial respiratory chain.[31] CoenzymeQ10 defects are associated with deficiency of CoQ10-independent mitochondrial respiratory chain complexes.[32] Genistein and CoQ10 exerted similar protective effects on MTPT and RCR in mitochondria. However, the underlying mechanism is unclear. Several studies indicated a possible relation of abnormal cellular CoQ10 to mitochondrial dysfunction, accompanied by oxidation of lipids/proteins and increased ROS production. Therefore, the similar protective effect of CoQ10 and genistein might be related to ROS clearance. However, the detailed mechanism requires further investigation.
Caspases are categorized as either pro-apoptotic or proinflammatory, according to individual participation in cellular programs. Caspase-1 is the major proinflammatory factor. The inflammasomes activation leads to cleavage of caspase-1 in response to danger signals. Caspase-1 activation control secretion of interleukins, such as IL-18 and IL-1b, whose potent proinflammatory activities direct infection and injury [33].Increased expression of caspase-1 was observed in lethally infected animal model[34].Caspase-8 has a pro-survival activity, which is resistant to necrosis. Andrew et al. reported caspase-8 prevents necrosis in RIPK3-dependent manner without inducing apoptosis. Genetic ablation of caspase-8 resulted in embryonic lethality in mice [35]. It was reported that mice were susceptible to spontaneous ileitis in the absence of caspase-8 and hence developed terminal ileitis with defects in antimicrobial host defense [36]. We found increased expression of caspase-1 and caspase-8 in liver necrosis (model group) while genistein attenuated the occurrence of necrosis, which is consistent with the above findings.
PCNA participated in DNA synthesis and was used to evaluate liver regeneration [37].In our study, the expression level of PCNA was higher in genistein co-treatment group than in the model group, indicating the regeneration in ACLF was attributed to genistein. Wang et al. reported that increased expression of PCNA improved survival rate in acute liver failure rats ,[38] which was consistent with our finding.
6CONCLUSIONSIn conclusion, our findings suggested that genistein, as an inhibitor of HIF-1α, might represent a novel strategy to attenuate liver failure in ACLF (as shown in Figure 4F). Future studies should explore detailed mechanisms underlying the treatment of ACLF with genistein.
7Authors, contributionsF. X. carried out the molecular biology studies, participated in the data analysis and drafted the manuscript. J.L.D, K.W., X.L. and D.C. performed the experiments. Y.Z. performed the statistical analysis. Q.H.M. is the corresponding author. All authors approved the final version.
8Financial SupportThis work was funded by the Natural Science Foundation of Beijing Municipal, China (7112064); Beijing Precision Medicine and Transformation Engineering Technology Research Centre of Hepatitis and Liver Cancer; Beijing Municipal Institute of Public Medical Research Development and Reform Pilot Project (2016-2);Foundation of Beijing Institute of Hepatology(No BJIH-01706); You’ an Foundation of Liver Disease and AIDS, China (BJYAH2016YN02).
9Conflict Of InterestThe authors declare that they have no conflicts of interest.