Liver injury caused by methotrexate (MTX) has mostly been investigated without applying criteria for the assessment of causality of drug induced liver injury (DILI). Hence, the existence of DILI by MTX in many cases is debatable. This study aimed to describe the frequency and characteristics of liver injury caused by MTX, applying DILI diagnostic criteria.
Material and methodsRheumatoid arthritis (RA) and psoriatic arthritis (PsA) patients who were treated with MTX in association with folic acid were included. Serial determinations of alanine amino transferase (ALT) and aspartate amino transferase (AST) were performed. The Roussel Uclaf Causality Assessment Method (RUCAM) was applied in cases of increases of ALT/AST over 1.5 upper limit of normal. Liver biopsy was considered when the total cumulative dosage (TCD) of MTX was ≥3.5g.
ResultsA total of 43 patients were analyzed (median follow up 32 (range: 1–48) months; 3.33 ALT/AST determinations per year). Five subjects presented an increase of ALT/AST. All presented a RUCAM score for MTX≤2 (improbable). Three had a RUCAM score for non-steroidal anti-inflammatory drugs ≥7 (probable) and two patients presented non-alcoholic fatty liver disease. Five patients with no other cause for liver disease consented to liver biopsy (TCD MTX: median 5.1; range: 3.5–7.4g). No significant fibrosis or steatosis was evident on histology.
ConclusionsNo biochemical or significant histological liver toxicity for MTX was demonstrated when applying causality criteria for DILI. More studies with this methodology are necessary in order to improve the assessment of its frequency.
Methotrexate (MTX) is a folic acid analog with anti-proliferative, immunosuppressive and anti-inflammatory activity used in the treatment of arthritis since 1951 [1]. At present, one of the most common indications of MTX is the management of rheumatoid arthritis (RA) and psoriatic arthritis (PsA). MTX has modified the natural history with significant improvement of quality of life of these patients [2–5].
Hepatotoxicity caused by MTX is second in frequency to gastrointestinal adverse effects [6]. With the use of single weekly low doses and the co-administration of folic acid, the frequency and severity of hepatotoxicity is reduced without loss of anti-inflammatory therapeutic effect [7–9].
A recent systematic review [10] of MTX-induced liver toxicity of 18 studies with a weekly mean dose of 12.5mg included 2199 patients. The cumulative incidence of elevated liver transaminases was 49%. However, only 24% received folic acid and 56% concomitantly received non-steroidal anti-inflammatory drugs (NSAIDs). Due to enzyme elevation, MTX was reduced or suspended in 26% and permanently discontinued in 7%. In thirty-three other studies, also included in this review, liver histology was analyzed in 2179 patients with an average total cumulative dosage (TCD) of 2.4g showing mild fibrosis in 15.3%, moderate to severe fibrosis in 1.3% and cirrhosis in 0.5%. In pre-treatment liver biopsy from 372 patients, fibrosis was present in 9.1% and cirrhosis in 0.3%. Only 9% received folic acid supplement. Worth mention is the fact that, in most studies, risk factors for pre-existing liver disease such as alcohol use, obesity and diabetes were insufficiently reported. Therefore, in most of the studies analyzed, one of the most important criteria to attribute hepatic injury to a given drug was not met, which is to rule out other associated causes that may provoke it [11].
This shortfall spiked the interest to study the hepatotoxicity by MTX, using the current diagnostic methodology of drug induced liver injury (DILI). The objective of this study is to describe the frequency and characteristics of DILI by MTX in patients with RA and PsA with long-term MTX therapy, combined with folic acid in patients seen in a Rheumatology department in Buenos Aires.
2Materials and methodsBetween December 2011 and September 2014, we included consecutive 18 years or older patients with diagnosis of RA (according to ACR/EULAR 2010 criteria [12]) or PsA (according to CASPAR criteria [13]) seen in the Rheumatology Department and receiving continuous weekly MTX treatment (oral or parenteral) up to 25mg, combined with folic acid. Patients with neoplasia or any condition with an expected survival under one year, those who could not comply with follow-up, who had not completed the initial evaluation, patients with Hepatitis B, Hepatitis C, HIV infection or autoimmune liver disease were excluded. This observational study was approved by the institutional review board and conformed to the ethical guidelines of the 2013 Declaration of Helsinki. All participants gave written consent.
Serial follow-up was carried out by hepatologists in order to determine the presence of MTX-induced hepatotoxicity. The initial evaluation consisted of a clinical history with anamnesis, physical exam, biochemical work-up and abdominal ultrasound. History of previous alcohol use, type, dose and duration of drug consumption, the TCD of MTX, non-alcoholic fatty liver disease associated factors (NAFLD) [14] such as obesity, diabetes, dyslipidemias and metabolic syndrome (according to the ALAD Consensus [15]) were recorded.
The diagnosis of liver steatosis was based on abdominal ultrasound and defined as: (1) presence of diffuse hyperechoic echotexture (bright liver), (2) increase of liver echogenicity compared with the kidneys, (3) vascular blurring, and (4) deep attenuation [16].
In the initial evaluation as in follow-up, alanine aminotransferase (ALT)/aspartate aminotransferase (AST) determinations as markers of MTX toxicity were measured. Increases of these enzymes over 1.5 times upper limit of normal (ULN) were assessed with the Roussel Uclaf Causality Assessment Method (RUCAM) model [17,18] in order to determine the presence or absence of toxicity by MTX or by other drugs. This system was specifically designed to determine the probability of a given drug developing liver toxicity based on: (1) the time relation between the administration of the drug and the onset of liver abnormalities; (2) the lapse to normalization after suspension; (3) the presence of risk factors such as alcohol consumption and age >55 years; (4) the consideration of other potential causes of enzyme alteration such as other drugs or concomitant liver disease and (5) behavior of the enzymes in case of re-exposure to the drug. A score of 0 or less rules out relation to the drug, 1–2 is improbable, 3–5 is possible, 6–8 is probable and >8 is highly probable.
Liver biopsy (with additional prior informed consent) was proposed to the patients who had a MTX TCD≥3.5g or concurrent liver disease or sustained hepatic enzyme abnormalities, according to the recommendations of MTX management [19–21]. The histological study was done with hematoxylin and eosin and Masson's trichrome stain. The histological scoring system of Kleiner et al. [22] was used considering it as the best adapted to alterations historically attributed to MTX [23–26]. It assesses histological findings such as hepatocellular ballooning, steatosis, lobular inflammation, hepatocellular injury and characteristics of fibrosis. Steatosis is divided into 4 grades according to the percentage of hepatocytes with fat vacuoles: Grade 0 or absent (<5%), 1 or mild (5–33%), 2 or moderate (>33–66%) and 3 or severe (>66%). The lobular inflammation is classified, according to the presence of foci of 2 or more inflammatory cells, into: Grade 0 or absent (no foci), 1 or mild (<2 foci per 200× field), 2 or moderate (2–4 foci per 200× field) and 3 or severe (>4 foci per 200× field). Fibrosis is divided into stages: Stage 1: perisinusoidal or periportal (1a: mild, perisinusoidal, zone 3; 1b: moderate, perisinusoidal, zone 3 and 1c: periportal or portal); Stage 2: perisinusoidal and periportal without bridges of fibrosis; Stage 3: presence of bridging fibrosis and Stage 4: cirrhosis.
2.1Statistical analysisQuantitative variables are expressed as mean±standard deviation if were normally distributed. Otherwise, they are presented as median and range. Qualitative results are expressed as percentages. The incidence rate of AST/ALT elevations was calculated considering the risk time from the baseline determination to the first elevation or to the last follow-up in cases with no liver enzyme elevation.
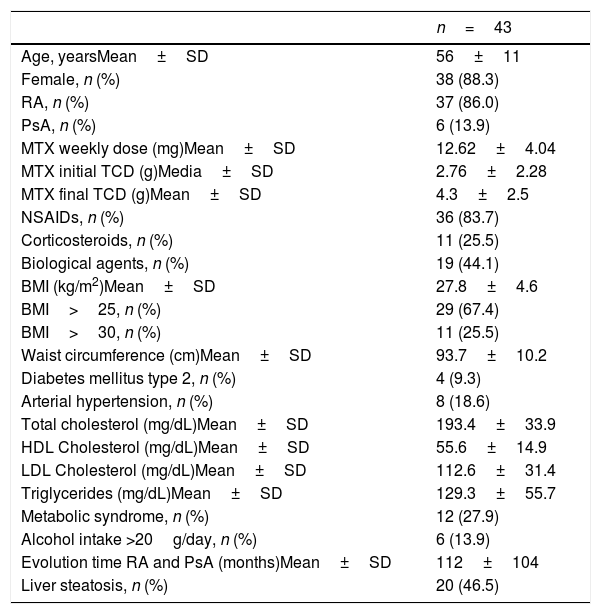
3ResultsA total of 43 patients were enrolled in this study. Clinical and biochemical features are shown in Table 1.
General features of patients enrolled.
| n=43 | |
|---|---|
| Age, yearsMean±SD | 56±11 |
| Female, n (%) | 38 (88.3) |
| RA, n (%) | 37 (86.0) |
| PsA, n (%) | 6 (13.9) |
| MTX weekly dose (mg)Mean±SD | 12.62±4.04 |
| MTX initial TCD (g)Media±SD | 2.76±2.28 |
| MTX final TCD (g)Mean±SD | 4.3±2.5 |
| NSAIDs, n (%) | 36 (83.7) |
| Corticosteroids, n (%) | 11 (25.5) |
| Biological agents, n (%) | 19 (44.1) |
| BMI (kg/m2)Mean±SD | 27.8±4.6 |
| BMI>25, n (%) | 29 (67.4) |
| BMI>30, n (%) | 11 (25.5) |
| Waist circumference (cm)Mean±SD | 93.7±10.2 |
| Diabetes mellitus type 2, n (%) | 4 (9.3) |
| Arterial hypertension, n (%) | 8 (18.6) |
| Total cholesterol (mg/dL)Mean±SD | 193.4±33.9 |
| HDL Cholesterol (mg/dL)Mean±SD | 55.6±14.9 |
| LDL Cholesterol (mg/dL)Mean±SD | 112.6±31.4 |
| Triglycerides (mg/dL)Mean±SD | 129.3±55.7 |
| Metabolic syndrome, n (%) | 12 (27.9) |
| Alcohol intake >20g/day, n (%) | 6 (13.9) |
| Evolution time RA and PsA (months)Mean±SD | 112±104 |
| Liver steatosis, n (%) | 20 (46.5) |
SD: standard deviation. RA: rheumatoid arthritis. PsA: psoriatic arthritis. MTX: methotrexate. TCD: total cumulative dosage. NSAIDs: non-steroidal anti-inflammatory drug. BMI: body mass index. HDL: high-density lipoprotein. LDL: low-density lipoprotein.
At baseline, 1 case (2.3%) which presented elevation of ALT/AST was classified as probable DILI caused by celecoxib (RUCAM score=8).
The follow-up of the 43 patients had a median of 32 (range: 1–48) months, and 3.3±1.7 biochemical tests – including liver enzymes – per year were performed. During this follow-up, 4 patients (9.3%) presented ALT/AST elevations (incidence rate: 3.9 cases per 100 persons-year). In 2 cases, the ALT/AST elevations were attributed to NSAIDs, 1 to meloxicam (RUCAM 7=probable) and 1 to the association of ibuprofen and diclofenac (RUCAM 7=probable). The remaining 2 cases met all criteria for NAFLD. Both presented steatosis on liver imaging and metabolic syndrome associated to fluctuating ALT/AST elevations which did not exceed 4 times ULN, in absence of a history of alcohol consumption.
In the 5 cases with ALT/AST elevations, the RUCAM for MTX score did not exceed 2 (Improbable).
Five patients consented to undergo liver biopsy. At the moment of biopsy, they presented a TCD for MTX between 3.5 and 7.4g (median 5.1) with no steatosis on ultrasound and no history of alcohol use. The histological findings can be seen in Fig. 1.
Two patients (Case 1 and Case 2) with TCD MTX of 4.2 and 4.7g respectively presented neither fibrosis nor steatosis. They presented only some non-specific cellular changes which did not correspond to any particular entity. These patients had no risk factors for liver disease.
One patient (Case 3) on corticosteroid therapy and who had a 5 month history of ALT/AST elevation attributed to celecoxib (mentioned previously) with a TCD MTX of 3.5g presented a histological study showing mild portal, perisinusoidal and perivenular fibrosis (Kleiner's Score Fibrosis 2) without bridging, steatosis, inflammation, hepatocellular ballooning or other changes that imply NAFLD or other entity.
Another patient (Case 4) with a TCD for MTX of 5.6g presented mild portal and perivenular fibrosis (Kleiner's Score Fibrosis 1c) and no other changes. After biopsy, this patient presented liver injury due to meloxicam (aforementioned).
The last case (Case 5), who had a TCD for MTX of 7.4g presented 5% steatosis and mild portal fibrosis (Kleiner's Score Fibrosis 1c). The patient was borderline according to the definition of NAFLD and had no histological findings of steatohepatitis. The patient presented central obesity with a normal body mass index and signs of muscle wasting associated to limited mobility due to RA.
4DiscussionMany studies have assessed MTX hepatotoxicity but few have applied diagnostic criteria for drug related causality. To our knowledge, this is the only study to have carried out a systematic evaluation of DILI in patients with chronic low-dose methotrexate and folic acid therapy. In clinical practice, diagnosis of DILI commonly implies a challenge since there is no specific diagnostic test [27]. Several score assessment methods have been developed. Currently, RUCAM scale, used in our study, is the most widely employed and recommended by experts. This method assesses the timing of onset of the adverse event in relation to starting the medication (challenge), and the timing of resolution in relation to stopping the medication (de-challenge), clinical features, specific risk factors, the exclusion of other transaminase-elevating conditions and the behavior after re exposure (re-challenge) if present. Points are awarded for each element and a final score confers the probability of a given drug's implication in causality of liver injury [28].
A relevant aspect which strengthens the applicability of these methods is the determination of other drugs with potential hepatotoxicity when combined with MTX. In a recent cross-sectional study [29] carried out in Buenos Aires on a series of 103 patients with RA and 15 with PsA (51% received MTX), the Maria and Victorino [30] system was applied to assess DILI. In 15 cases (12.5%), all liver injury was attributed to NSAID use, mainly diclofenac. In patients with RA and PsA on multiple drug treatment, singling out which drug is implied in hepatotoxicity requires a step-by-step method to determine the responsible agent [11,27].
In the present study, carried out on 43 patients with a median follow-up greater than 2.5 years, no MTX hepatotoxicity was detected using the diagnostic methodology mentioned. The ALT/AST elevations were attributed to NSAIDs in 3 cases (6.9%) and to NAFLD in 2 cases (4.6%). On occasion, it can be thought that the ALT/AST elevations detected in some studies and in clinical practice have no relation to MTX. An erroneous etiological interpretation may lead to the discontinuation or definitive suspension of MTX, an effective, easily administered, low-cost drug and its replacement with alternative drugs such as costly biological agents with serious adverse effects. Some guidelines recommend contraindication or suspension of MTX according to the different levels or persistence of ALT/AST elevations without a diagnostic algorithm or an expert opinion to assess whether MTX was the probable cause of hepatotoxicity [2,19–21,31–33].
The changes attributed to MTX in liver histology are indistinguishable from those caused by other entities such as alcohol-induced liver disease, NAFLD and secondary hepatic steatosis [26]. In presence of other entities which potentially induce liver injury, it is difficult to claim MTX as the cause of the histological findings. Moreover, most studies which include histological assessment apply classifications which consider the type of fibrosis observed in chronic viral hepatitis which elicit fibrosis starting at the portal space and, from there, spreading to the remaining parenchyma [34,35]. Hence, the perisinusoidal NAFLD-like characteristics of presumptive MTX fibrosis are not adequately assessed. We chose Kleiner's classification [22] given its greater precision to assess changes attributable to MTX as well as other associated conditions commonly seen in patients with RA [36]. In the present study, no significant histological liver toxicity was found. In 5 patients with TCD (equivalent for time of exposure to injury) greater or equal to 3.5g of MTX and with no other evident cause of liver disease, no significant histological changes were found after years of treatment, including one case of 12 year-long treatment.
The low percentage of ALT/AST elevations compared to those observed in other studies included in the systematic review by Visser and van der Heijde [10] might be explained by the low frequency of alcohol consumption and obesity in the sample and the use of folic acid among all our patients.
In this small series, no biochemical or significant histological liver toxicity for MTX was demonstrated when applying causality criteria for DILI. There is no decisive evidence indicating MTX hepatotoxicity, especially when associated with folic acid and in weekly doses no greater than 25mg, commonly used in treatment of RA, PsA and inflammatory intestinal diseases. Further studies with larger population samples using DILI diagnostic methodology and the inclusion of experts trained in liver disease are needed to enhance knowledge of the causality or absence of MTX hepatotoxicity.
AbbreviationsMTX
methotrexate
RArheumatoid arthritis
PsApsoriatic arthritis
DILIdrug induced liver injury
ALTalanine aminotransferase
ASTaspartate aminotransferase
NAFLDnon-alcoholic fatty liver disease
TCDtotal cumulative dosage
RUCAMRoussel Uclaf Causality Assessment Method
NSAIDsnon-steroidal anti-inflammatory drugs
ULNupper limit of normal
Grants and financial supportNothing to disclose.
Conflicts of interestNone.