Acute liver failure (ALF) is a severe disease which is associated with a high mortality rate. As mild hypothermia has been shown to have protective effects on the brain, this study aimed to determine whether it also provides protection to the liver in rats with ALF and to explore its underlying mechanism.
Materials and methodsIn total, 72 rats were divided into 3 groups: control group (CG, treated with normal saline), normothermia group (NG, treated with d-galactosamine and lipopolysaccharide; d-GalN/LPS), and mild hypothermia group (MHG, treated with d-GalN/LPS and kept in a state of mild hypothermia, defined as an anal temperature of 32–35°C). The rats were examined at 4, 8, and 12h after treatment.
ResultsMild hypothermia treatment significantly reduced serum alanine transaminase and aspartate transaminase levels and improved the liver condition of rats with d-GalN/LPS-induced ALF at 12h. Serum tumor necrosis factor-alpha levels were significantly lower in the MHG than in the NG at 4h, but no significant differences were observed in the interleukin-10 levels between the NG and MHG at any time. The serum and hepatic levels of high mobility group box 1 were significantly lower in the MHG than in the NG at 8 and 12h. The protein expression levels of cytochrome C and cleaved-caspase 3 in hepatic tissues were significantly lower in the MHG than in the NG at 8h.
ConclusionMild hypothermia improved the liver conditions of rats with ALF via its anti-inflammatory and anti-apoptotic effects.
Acute liver failure (ALF) is a severe liver disease that manifests with massive hepatic necrosis [1]. It is characterized by rapid loss of liver function with severe jaundice, coagulopathy, hepatic encephalopathy, and eventual multiple organ failure, which is responsible for its poor prognosis and the associated high mortality [1,2]. Although liver transplantation is currently the most effective treatment for ALF, it is limited by donor shortage, high cost, and life-threatening complications [3]. Therefore, it is necessary to explore new treatment options for ALF.
Immune-mediated injury involving multiple immune cells and inflammatory factors plays a critical role in the occurrence and development of ALF [4]. As a crucial pro-inflammatory factor associated with hepatic injury, tumor necrosis factor-alpha (TNF-α) promotes inflammatory response in a variety of ways, such as by stimulating the production of nitric oxide and reactive oxygen species [5]. In contrast, interleukin (IL)-10 inhibits the production of pro-inflammatory factors such as TNF-α and IL-12, and negates their inflammatory responses [6]. Further, high mobility group box 1 (HMGB1), released from necrotic cells following endogenous injury, activates monocytes and macrophages. Extracellular HMGB1 can cause mononuclear cells to produce a variety of cytokines and promote initial activation, proliferation, and differentiation of T-cells, which are involved in immune-mediated hepatic injury [7,8]. In addition to necrosis, overactivation of hepatic apoptosis plays an important role in early-stage death of massive hepatocytes [9]. Apoptosis signal transduction mainly occurs via the mitochondrial pathway (intrinsic pathway) and the death receptor pathway (extrinsic pathway) involving cytochrome C (Cyt C) release from the mitochondria, binding of death receptors with ligands, and caspase cascade reaction, in which cleaved-caspase 3 is considered a key apoptotic “executioner” enzyme in mammalian cells [10,11].
Mild hypothermia has been shown to have protective effects on the brain under clinical conditions such as cerebral infarction and cardiac arrest [12,13]. It has also been found to afford some protective effects to other organs, such as the heart and kidney [14]. Previous studies on patients with ALF showed that although mild hypothermia (anal temperature of 32–35°C) could effectively improve neurological complications such as cerebral edema and intracranial hypertension [15,16], its exact effects on hepatic injury are still unclear. In this study, we aimed to determine whether mild hypothermia affords protection to the liver of rats with ALF and to explore the underlying mechanism of any such protective effects.
2Materials and methods2.1Animal modelsSeventy-two 7–8-week-old female Sprague-Dawley rats (weighing 200–250g each) were purchased from the Experimental Animal Center of Nanchang University and placed in the laboratory with access to food and water for a week at a temperature of 23–25°C and 60–70% humidity. Access to food was withheld from the rats 12h before the experiments, and the rats were then randomly divided into a control group (CG, n=24), normothermia group (NG, n=24), and mild hypothermia group (MHG, n=24). ALF was induced in the experimental groups using d-galactosamine (d-GalN; 400mg/kg; intraperitoneally, i.p.) and lipopolysaccharide (LPS; 100μg/kg, i.p.) [17]. The CG rats were only provided equal volumes of normal saline. After d-GalN/LPS treatment, mild hypothermia was induced by alcohol spray and the directional cool wind method in the MHG [18]. The anal temperature of rats in the other two groups was monitored and maintained at 37°C using a heater. All animal experimental procedures and protocols were approved by the Medical Research Committee of The First Affiliated Hospital of Nanchang University (ethics number: 2010, medical research no. 50) and were in accordance with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health.
2.2Sample collectionAfter treatment, the rats were anesthetized using 10% chloral hydrate (300mg/kg) i.p. at 4, 8, and 12h (eight rats for each time point). Blood samples were collected from the postcaval vein after exposing the abdominal cavity. A 4-mL blood sample from each rat was taken into a serum tube and centrifuged at 12,000g for 10min at 4°C. The supernatant was used to examine the serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), TNF-α, IL-10, and HMGB1. Following exsanguination of the rats under deep anesthesia with chloral hydrate, the livers were exposed. The right hepatic lobe weighing 50–100mg was excised, placed in a frozen storage tube, and immediately stored in liquid nitrogen for later measurement of the levels of HMGB1, Cyt C, and caspase 3. The rest of the liver samples were fixed with cold 10% neutral formaldehyde for histopathological and immunohistochemical (IHC) examinations.
2.3Liver function testsThe serum ALT and AST levels of the rats were measured [19] using the Hitachi 7600 automatic biochemical analyzer system (Hitachi Co. Ltd., Tokyo, Japan).
2.4Enzyme-linked immunosorbent assay (ELISA)Serum TNF-α, IL-10, and HMGB1 levels were determined using the ELISA method with ELISA kits (Xitang Biotechnology Co. Ltd., Shanghai, China), according to the manufacturer's instructions.
2.5Reverse transcription-polymerase chain reaction (RT-PCR)The mRNA expression levels of HMGB1 in hepatic tissues were determined using RT-PCR [20]. The total ribonucleic acid (RNA) was extracted from the frozen hepatic tissue using RNA Trizol reagent (Beijing TransGen Biotechnology Co. Ltd., Beijing, China). The cDNA was then synthesized with 1μg RNA using a reverse transcriptase kit (Tiangen Biotechnology Co. Ltd., Beijing, China), and PCR was performed with 1μL cDNA using a PCR kit (Tiangen Biotechnology Co. Ltd., Beijing, China), according to the manufacturer's instructions. β-Actin was used as a control. Finally, the PCR products were separated using 1.2% agarose gel electrophoresis and the strip was analyzed using the gel analysis system. The gray value ratio of HMGB1/β-actin, which indicates the relative mRNA expression levels of HMGB1, was calculated. The sequences of the primers used in this study were as follows: HMGB1-5′-GATGACAAGCAGCCCTAT-3′ (forward) and 5′-TCCATGCC AATTTACAAC-3′ (reverse), 481 bps; β-actin-5′-CCAACCGTGAAAAGATGACC-3′ (forward) and 5′-CAGGAGGAGCAATGATCTTG-3′ (reverse), 660 bps.
2.6Western blot analysisTotal and cytoplasmic proteins were extracted using the radioimmunoprecipitation assay lysis buffer (containing the protease inhibitor phenylmethylsulfonyl fluoride [PMSF]) and cytoplasm buffer (containing the phosphatase inhibitor sodium fluoride [NaF], PMSF, and dithiothreitol) (Sangon Biotechnology Co. Ltd., Shanghai, China). Protein concentrations were then determined using the bicinchoninic acid protein quantitative kit (Vazyme Biotechnology Co. Ltd., Nanjing, China), according to the manufacturer's instructions. The protein samples were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes. After blocking with 5% milk at room temperature (RT) for 1h, the membranes were incubated with primary antibodies (anti-Cyt C, anti-pro-caspase 3, anti-cleaved-caspase 3 or anti-β-actin) (Boster Biotechnology Co. Ltd, Wuhan, China) at 4°C overnight and then incubated with horseradish peroxidase-conjugated secondary antibody-horse anti-rabbit immunoglobulin (IgG) (Boster Biotechnology Co. Ltd, Wuhan, China) at 37°C for 1h. Finally, protein bands were visualized with enhanced chemiluminescence (Applygen Technologies Co. Ltd, Beijing, China) and analyzed by Gel-Pro analyzer 4.0. The gray value ratio of target proteins/β-actin was calculated.
2.7Liver histology and immunohistochemistryThe fixed hepatic tissues were embedded with paraffin and sliced into 4-μm sections. Some sections were stained with hematoxylin and eosin, dehydrated with alcohol, cleared with xylene, and mounted, and the histological characteristics were determined by light microscopy [21]. The other sections were used to detect the protein expression of HMGB1 using the IHC method. After deparaffination, hydration, and antigen retrieval, the paraffin-embedded sections were blocked with goat serum at RT for 1h and incubated overnight with anti-HMGB1 (Boster Biotechnology Co. Ltd, Wuhan, China) at 4°C. The sections were then incubated with horseradish peroxidase-conjugated secondary antibody-horse anti-rabbit IgG (Boster Biotechnology Co. Ltd, Wuhan, China) at 37°C for 1h and visualized with diaminobenzidine tetrahydroxychloride solution (Sigma, St. Louis, MO, USA), and brown staining was regarded a positive result. Five different areas of each slide were observed at ×400 magnification. IHC staining evaluation was based on the intensity of staining and range of positive cells [22]. The scoring for staining intensity was as follows: 0 for no staining, 1 for weak staining, 2 for medium staining, and 3 for strong staining, whereas the scoring for range was as follows: 1 for <10%, 2 for 10–50%, 3 for 50–80%, and 4 for 80–100%. The final staining score was calculated as staining degree×staining range.
2.8Statistical analysisStatistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Comparisons for more than two groups were performed by two-way analysis of variance followed by post hoc Bonferroni tests [23]. All quantitative data were presented as mean±standard deviation, and P<0.05 was considered statistically significant.
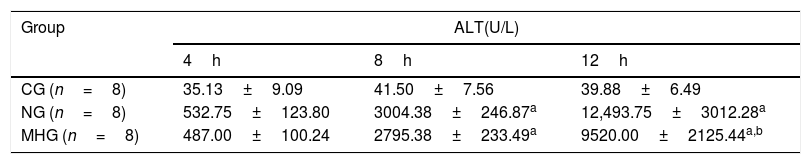
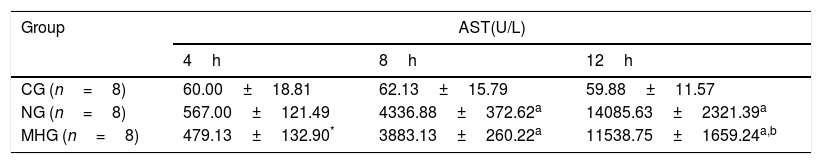
3Results3.1Mild hypothermia improved liver conditions in rats with ALFAs shown in Tables 1 and 2, serum ALT and AST levels were significantly higher in the NG and MHG than in the CG at all time points except at 4h (4h, P>0.05; 8h and 12h, P˂0.001), and they increased over time (4–12h). Further, serum ALT and AST levels were significantly lower in the MHG than in the NG at 12h (P˂0.001).
Effects of mild hypothermia on serum ALT level in rats treated with d-GalN/LPS.
| Group | ALT(U/L) | ||
|---|---|---|---|
| 4h | 8h | 12h | |
| CG (n=8) | 35.13±9.09 | 41.50±7.56 | 39.88±6.49 |
| NG (n=8) | 532.75±123.80 | 3004.38±246.87a | 12,493.75±3012.28a |
| MHG (n=8) | 487.00±100.24 | 2795.38±233.49a | 9520.00±2125.44a,b |
Note: Values were presented as mean±SD; n=8 for each time point. d-GalN, d-galactosamine; LPS, lipopolysaccharide; ALT, alanine aminotransferase; CG, control group; NG, normothermia group; MHG, mild hypothermia group; SD, standard deviation.
Effects of mild hypothermia on serum AST level in rats treated with d-GalN/LPS.
| Group | AST(U/L) | ||
|---|---|---|---|
| 4h | 8h | 12h | |
| CG (n=8) | 60.00±18.81 | 62.13±15.79 | 59.88±11.57 |
| NG (n=8) | 567.00±121.49 | 4336.88±372.62a | 14085.63±2321.39a |
| MHG (n=8) | 479.13±132.90* | 3883.13±260.22a | 11538.75±1659.24a,b |
Note: Values were presented as mean±SD; n=8 for each time point. d-GalN, d-galactosamine; LPS, lipopolysaccharide; ALT, alanine aminotransferase; CG, control group; NG, normothermia group; MHG, mild hypothermia group; SD, standard deviation.
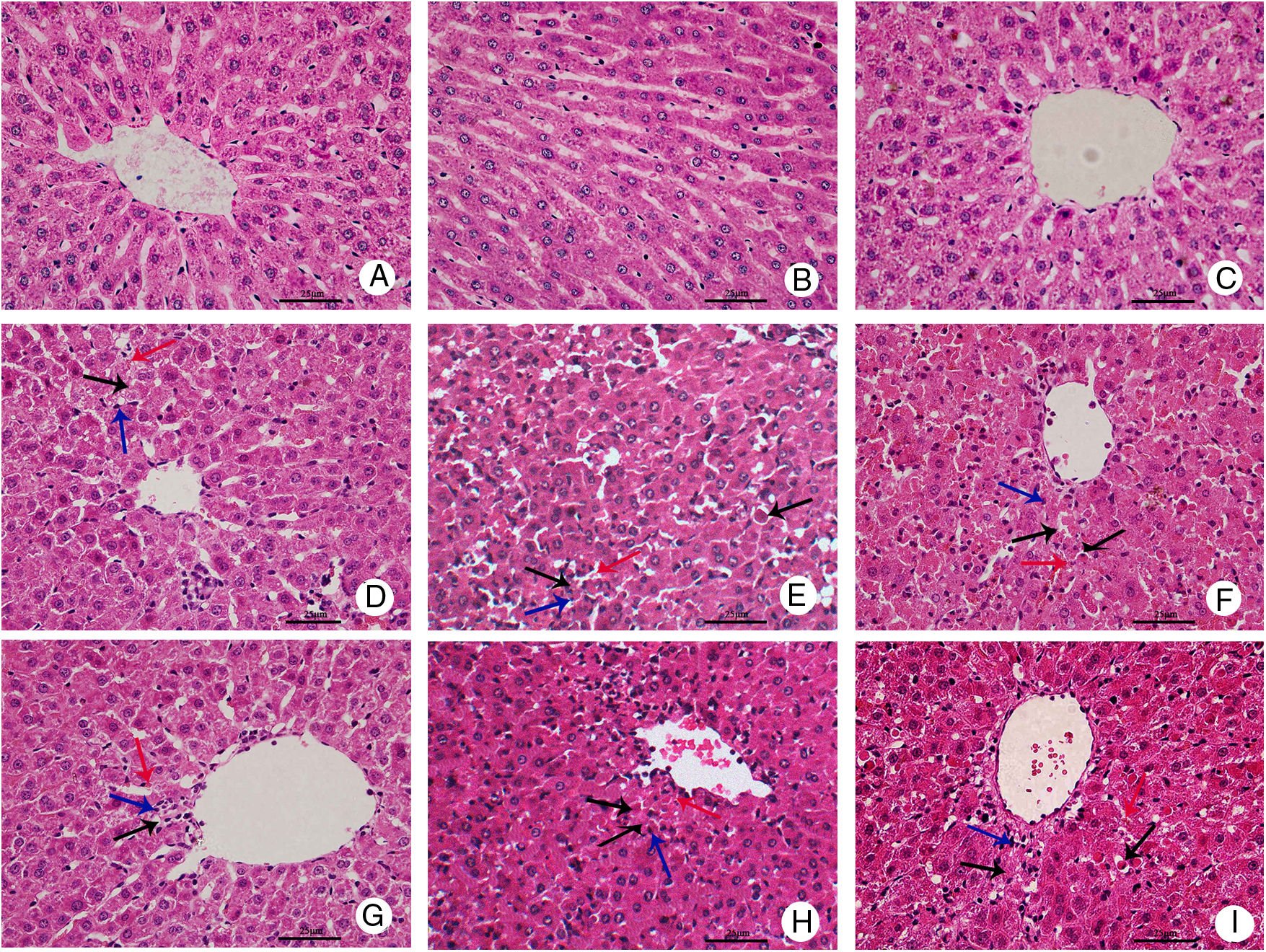
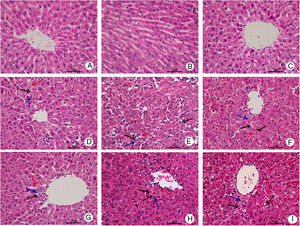
Representative histopathological slides of hepatic tissues are shown in Fig. 1. Hepatic tissue slides from the CG rats showed normal and complete structures of hepatic lobules, with no significant hepatic necrosis, hemorrhage, or inflammatory cell infiltration. In the hepatic tissue slides from the NG and MHG rats, normal structures of hepatic lobules were still observed at 4h after treatment, but partial hepatic cords fused into strips or flakes and hepatocytes showed ballooning degeneration, eosinophilic degeneration, and focal necrosis. Infiltration of a few inflammatory cells and minor congestion were also present in the hepatic sinus. At 8h, hepatic lobules showed disorganized structures, hepatic cords were dissociated, and some areas showed patchy hepatic necrosis. Apoptotic cells, congestion, and hemorrhage were also found in the hepatic sinus, and inflammatory cell infiltration significantly increased. At 12h, massive hepatic necrosis and loss of normal hepatic lobule structures were observed. A large amount of inflammatory cell infiltration into periportal and necrotic regions, and massive hemorrhage in the hepatic sinus were also noted. In contrast, the degree of hepatic injury was less severe in the MHG than in the NG at 12h. Lesser hepatic necrosis, less cellular debris, and infiltration of fewer inflammatory cells were observed in the MHG rats at 12h, but there were no significant differences at 4 or 8h.
Mild hypothermia improved liver conditions of rats with ALF hepatic tissues in each group were harvested at 4, 8, and 12h for histopathological examination (hematoxylin–eosin staining, 400× magnification); control group at (A) 4h, (B) 8h, and (C) 12h; normothermia group at (D) 4h, (E) 8h, and (F) 12h; mild hypothermia group at (G) 4h, (H) 8h, and (I) 12h. ALF, acute liver failure.
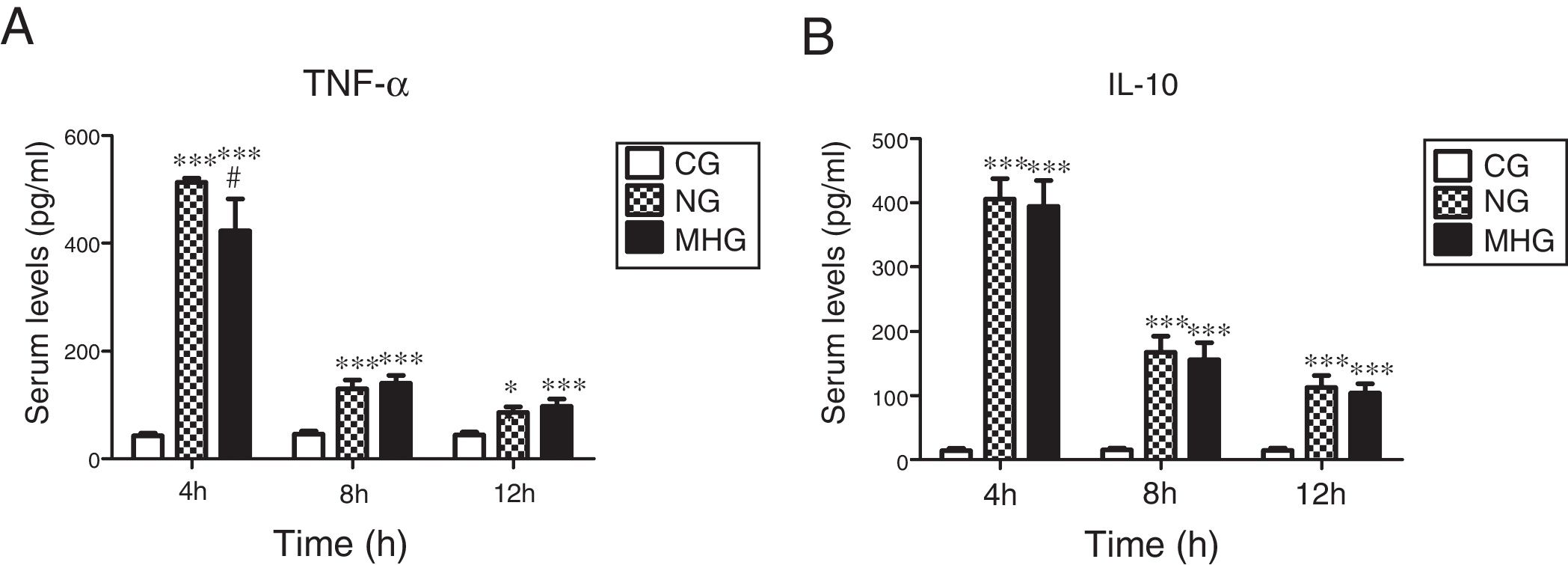
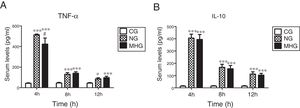
As shown in Fig. 2A and B, serum TNF-α and IL-10 levels were significantly higher in the NG and MHG than in the CG at all time points (P˂0.01 for all). Further, serum TNF-α levels were significantly lower in the MHG than in the NG at 4h (P˂0.05). However, no significant differences in the IL-10 levels were observed between the NG and MHG at any time point.
Effects of mild hypothermia on pro-inflammatory factor TNF-α and anti-inflammatory factor IL-10 (A) and (B) serum TNF-α and IL-10 levels determined by ELISA. Data are presented as mean±SD. ***P<0.001 vs. CG; **P<0.01 vs. CG; #P<0.001 vs. NG. TNF-α, tumor necrosis factor-alpha; IL-10, interleukin-10; ELISA, enzyme-linked immunosorbent assay; SD, standard deviation; CG, control group; NG, normothermia group; MHG, mild hypothermia group.
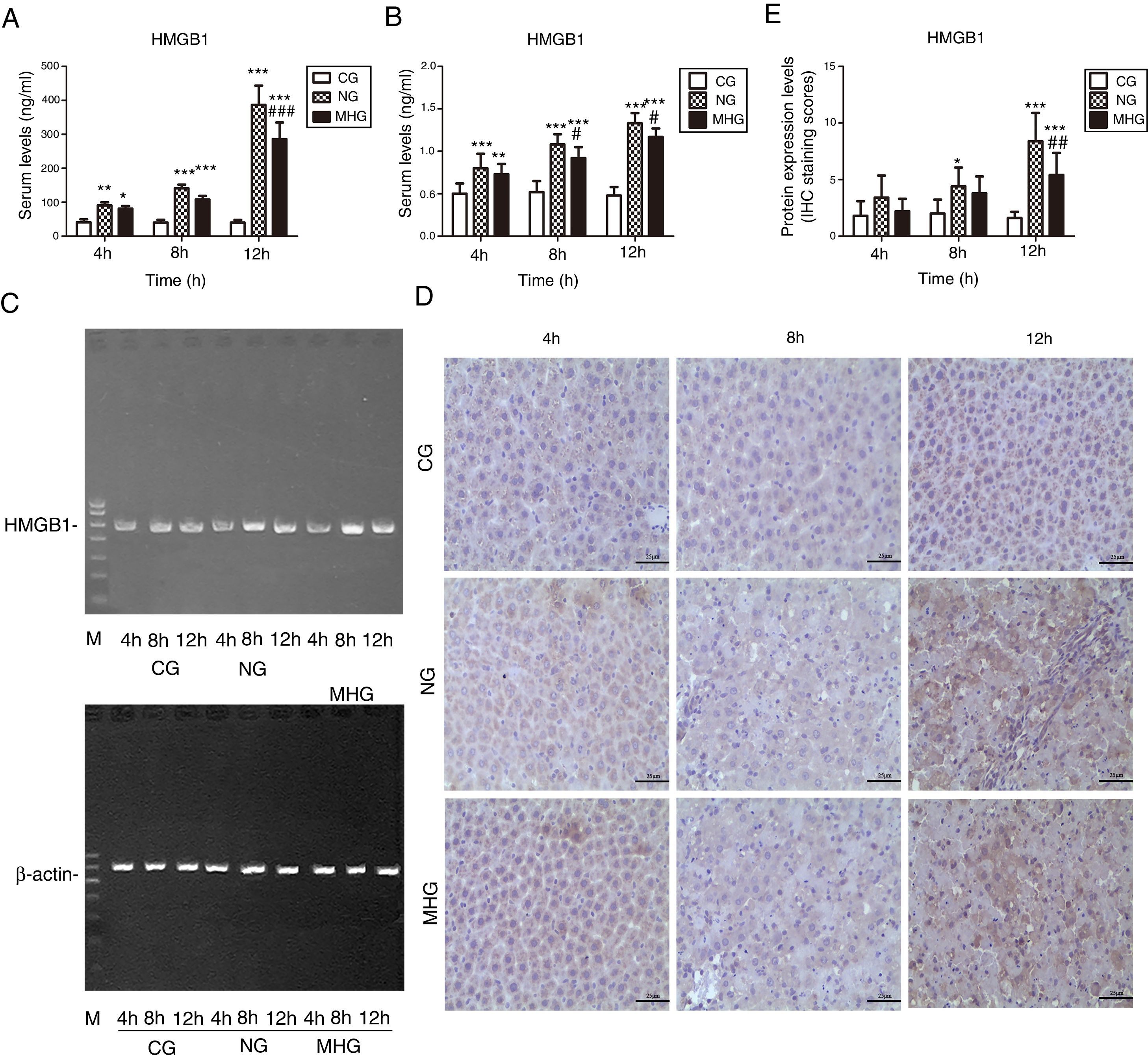
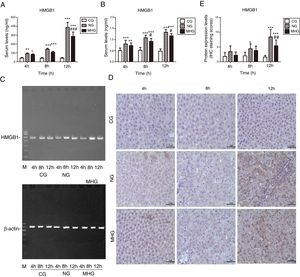
As shown in Fig. 3A, serum HMGB1 levels were significantly higher in the NG and MHG than in the CG at all time points (P<0.05 for all), and they increased over time, which was consistent with the RT-PCR results (P<0.05 for all) (Fig. 3B and C). Further, serum and mRNA expression levels of HMGB1 were significantly lower in the MHG than in the NG at 8 and 12h (P<0.05 for both).
Effects of mild hypothermia on HMGB1 levels in blood and hepatic tissues (A) and (B) serum HMGB1 levels determined by ELISA and mRNA levels of HMGB1 in hepatic tissues detected by RT-PCR. (C) Agarose gel electrophoresis images of HMGB1 mRNA expression in hepatic tissue. (D) Representative images of protein expression levels of HMGB1 in hepatic tissue, detected by immunohistochemistry at 400× magnification. (E) Protein expression levels of HMGB1 in hepatic tissue, indicated by immunohistochemistry staining scores. β-Actin was used as a control. ***P<0.001 vs. CG; **P<0.01 vs. CG; *P<0.05 vs. CG; ###P<0.001 vs. NG; ##P<0.01 vs. NG; #P<0.05 vs. NG. HMGB1, high mobility group box 1; ELISA, enzyme-linked immunosorbent assay; mRNA, messenger ribonucleic acid; RT-PCR, reverse transcription-polymerase chain reaction; CG, control group; NG, normothermia group; MHG, mild hypothermia group.
IHC examination showed only few light brown granules in the nucleus and cytoplasm in the CG. In contrast, more punctate light brown granules were found in the cytoplasm in the NG and MHG, which increased and deepened over time and finally merged to form patchy brown-stained areas (Fig. 3D). Staining scores showed that the protein expression levels of HMGB1 were low at all time points in the CG but increased over time in the NG and MHG (Fig. 3E). Further, the brown-stained areas were significantly smaller in the MHG than in the NG at 12h, which was also consistent with the staining score results (P<0.01).
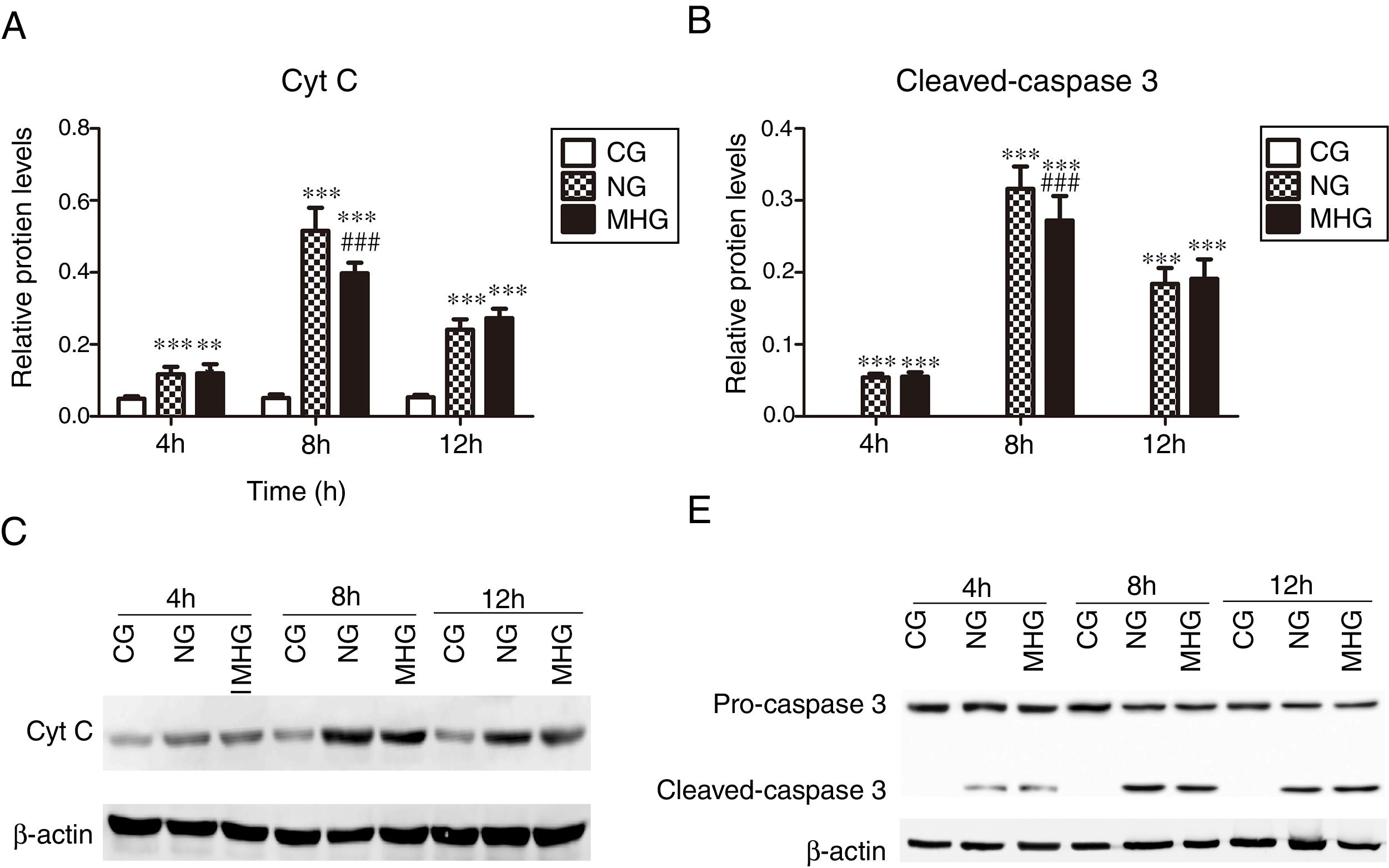
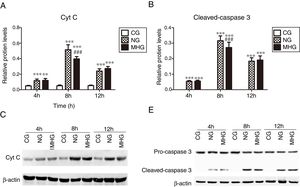
3.4Effects of mild hypothermia on protein expression of Cyt C and cleaved-caspase 3 in hepatic tissuesAs shown in Fig. 4 A and B, the protein expression levels of Cyt C and cleaved-caspase 3 were significantly higher in the NG and MHG than in the CG at all time points (P<0.001 for all). Further, the protein expression levels of Cyt C and cleaved-caspase 3 were significantly lower in the MHG than in the NG at 8h (P<0.001 for both), but no significant differences were observed at 4 and 12h. The representative Western blot images of Cyt C and cleaved-caspase 3 are shown in Fig. 4C and D.
Effects of mild hypothermia on the protein expression of Cyt C and Caspase 3 in hepatic tissues and (B) protein expression levels of Cyt C and cleave-caspase 3, detected by Western blot. (C and D) Representative Western blot images of Cyt C and caspase 3. β-Actin was used as a control. ***P<0.001 vs. CG; ###P<0.001 vs. NG. Cyt C, cytochrome C; CG, control group; NG, normothermia group; MHG, mild hypothermia group.
ALF is a rapidly aggravating disease with a poor prognosis and is associated with a high mortality [2]. Mild hypothermia has been shown to have protective effects on the brain [24,25]. Our study findings provide evidence that mild hypothermia improves liver conditions in rats with ALF and promote an understanding of the protective mechanisms of mild hypothermia.
As the morphological characteristics of ALF induced by d-GalN/LPS are similar to those of clinically encountered ALF [17], we designed a d-GalN/LPS-induced ALF model for our study to evaluate the effects of mild hypothermia on hepatic injury. We confirmed that the serum levels of ALT and AST increased and the histopathological changes worsened over time following the administration of d-GalN/LPS. However, after mild hypothermia treatment, serum ALT and AST levels decreased and advanced hepatic tissue injuries improved, demonstrating the protective effects of mild hypothermia in d-GalN/LPS-induced acute hepatic injury.
We further investigated the protective mechanisms of mild hypothermia in d-GalN/LPS-induced acute hepatic injury. Previous studies have shown that mild hypothermia can exert immune regulatory effects by affecting the functions of multiple inflammatory cells and through the production of inflammatory factors [26,27]. It can also inhibit apoptosis signal pathways at multiple levels, for example, by decreasing p53 levels [24,28]. Therefore, we speculated that the protective mechanisms of mild hypothermia in d-GalN/LPS-induced acute hepatic injury were associated with its anti-inflammatory and anti-apoptotic effects. Upon examination, we found significant increases in the serum levels of TNF-α and IL-10, which are critical pro- and anti-inflammatory factors, respectively. We also discovered that mild hypothermia inhibited the increase in early-phase serum TNF-α levels; however, no significant effect was observed on the serum IL-10 level, which is consistent with the findings of previous reports that showed that mild hypothermia did not affect IL-10 production by LPS-stimulated monocytes in vitro[29]. This indicates that the anti-inflammatory effects of mild hypothermia are associated with the inhibition of secretion of pro-inflammatory TNF-α and not the promotion of secretion of anti-inflammatory IL-10.
HMGB1 can be released from massive necrotic hepatocytes and activated monocytes and macrophages, which amplify the inflammatory response [7,30]. In this study, we determined the role of HMGB1 changes in the protective functions of mild hypothermia in d-GalN/LPS-induced acute hepatic injury. Previous studies have shown that HMGB1 plays an important role in ALF and could be an important predictor of the degree of hepatic injury in ALF [31,32]. We verified that HMGB1 levels in blood and liver tissues were significantly elevated in rats with ALF and gradually increased with the deterioration of hepatic injury but reversed after mild hypothermia treatment, which is consistent with the findings of previous reports that showed that lower serum HMGB1 levels were observed after mild hypothermia treatment in rats with sepsis [33]. The findings indicated that ALF involved upregulation of HMGB1 expression, and that the protective effects of mild hypothermia on the liver in d-GalN/LPS-induced acute hepatic injury involved downregulation of HMGB1 expression.
Cyt C is mainly present in the mitochondria and overflows from the mitochondria into the cytoplasm when stimulated by the apoptosis signal, which is the key process of the mitochondrial pathway [11]. As a pro-apoptotic protein, caspase 3 can be activated by both the death receptor pathway and the mitochondrial pathway to execute the apoptosis process [34]. To clearly understand the apoptotic changes in the liver, the protein expression levels of Cyt C and cleaved-caspase 3 were examined. As expected, the levels significantly increased after d-GalN/LPS administration and were inhibited by mild hypothermia at 8h, indicating that the anti-apoptotic effects of mild hypothermia were associated with inhibition of Cyt C release and activation of caspase 3. Further, TNF-α, the main ligand of death receptors, can induce hepatocyte apoptosis mediated by tumor necrosis factor receptor 2, which is associated with the release of Cyt C and the activation of caspase 3 [35,36]. In this study, we showed that mild hypothermia reduced the serum levels of TNF-α, which was consistent with the changes in Cyt C and cleaved-caspase 3 expressions. Further investigations should be conducted to determine whether the effects of mild hypothermia on the changes in Cyt C and cleaved-caspase 3 expressions are mediated by TNF-α.
In conclusion, we showed that inflammation and apoptosis are involved in ALF and that the protection afforded by mild hypothermia to the liver of rats with ALF may be attributed to the anti-inflammatory and anti-apoptotic effects exerted by the treatment.AbbreviationsALF acute liver failure d-galactosamine lipopolysaccharide control group normothermia group mild hypothermia group alanine transaminase aspartate transaminase tumor necrosis factor-alpha interleukin high mobility group box 1 cytochrome C immunoglobulin room temperature phenylmethylsulfonyl fluoride sodium fluoride immunohistochemical enzyme linked immunosorbent assay reverse transcription polymerase chain reaction
This work was supported by the Science and Technology Research Project of Jiangxi Provincial Education Department (Grant number: GJJ09113).
Conflict of interestThe authors have no conflicts of interest to declare.