SerpinB3 is a cysteine protease inhibitor involved in several biological activities. It is progressively expressed in chronic liver disease, but not in normal liver. The role in vascular reactivity of this serpin, belonging to the same family of Angiotensin II, is still unknown. Our aim was to evaluate the in vivo and in vitro effects of SerpinB3 on systemic and splanchnic hemodynamics.
Material and methodsDifferent hemodynamic parameters were evaluated by ultrasonography in two colonies of mice (transgenic for human SerpinB3 and C57BL/6J controls) at baseline and after chronic carbon tetrachloride (CCl4) treatment. In vitro SerpinB3 effect on mesenteric microvessels of 5 Wistar-Kyoto rats was analyzed measuring its direct action on: (a) preconstricted arteries, (b) dose–response curves to phenylephrine, before and after inhibition of angiotensin II type 1 receptors with irbesartan. Hearts of SerpinB3 transgenic mice and of the corresponding controls were also analyzed by morphometric assessment.
ResultsIn SerpinB3 transgenic mice, cardiac output (51.6±21.5 vs 30.1±10.8ml/min, p=0.003), hepatic artery pulsatility index (0.85±0.13 vs 0.65±0.11, p<0.001) and portal vein blood flow (5.3±3.2 vs 3.1±1.8ml/min, p=0.03) were significantly increased, compared to controls. In vitro, recombinant SerpinB3 had no direct hemodynamic effect on mesenteric arteries, but it increased their sensitivity to phenylephrine-mediated vasoconstriction (p<0.01). This effect was suppressed by inhibiting angiotensin II type-1 receptors.
ConclusionsIn transgenic mice, SerpinB3 is associated with a hyperdynamic circulatory syndrome-like pattern, possibly mediated by angiotensin receptors.
In cirrhosis, portal hypertension results from an increase in the intrahepatic resistance to portal flow due to liver fibrosis and alteration in intrahepatic vasculature. Portal hypertension is also caused by an increase in portal blood inflow, due to increased splenic and mesenteric blood flow caused by the vasodilation of splanchnic arteries [1]. Systemic and splanchnic vasodilation is the main cause of the onset of the hyperdynamic circulatory syndrome of cirrhosis, characterized by increased cardiac output and heart rate, decreased systemic vascular resistance and low arterial blood pressure [1].
According to the “peripheral arterial vasodilation theory” [1], splanchnic vasodilation leads to renal sodium retention and, as a consequence, hypervolemia which contributes to the hyperdynamic syndrome by increasing cardiac preload. The hyperdynamic syndrome, in turn, maintains and enhances portal hypertension. The hyperdynamic circulatory syndrome is pivotal for the development of several complications of cirrhosis [1,2]. The leading mechanism for the development of this hemodynamic syndrome seems to be the altered sensitivity to vasoconstrictors and the increased effect of vasodilators [3].
SerpinB3 (SB3) is a member of the ov-serpins/clade B family, protease inhibitors involved in multiple biological functions and cell homeostasis control [4]. This serpin is physiologically expressed in squamous epithelium [4], in sweat glands, in endothelial cells of the veins and in arteries walls [5]. SB3 is not detectable in normal hepatocytes [6], while its expression progressively increases in chronic liver disease, cirrhosis and hepatocellular carcinoma [7]. A direct correlation of this molecule with Transforming Growth Factor-β1 (TGF-β1) expression has been reported in chronic hepatitis and hepatocellular carcinoma [8] and in experimental models of lung fibrogenesis in SB3 transgenic mice [9]. Moreover, it has been recently observed that SB3 over-expression significantly increases the expression of pro-fibrogenic genes, and activates hepatic stellate cells, favoring collagen deposition, in two murine models of liver fibrosis [10]. SB3 has an anti-apoptotic effect [11] and, in addition, it induces epithelial to mesenchymal transition and interleukin-6 (IL-6) expression as a consequence of unfolded protein response [11]. The involvement of this serpin in the control of proteolytic processes has important implications in fibrogenesis and neoplastic transformation, whereas the balance between proteases and their inhibitors can affect the extracellular matrix components, cell motility, invasiveness, proliferation and apoptosis [11].
Taking into account that members of the serpin family, such as Angiotensin II, are known to be involved in the regulation of arterial resistance [12] and are able to stimulate cell proliferation [13] and TGF-β1 and fibronectin gene expression [14], we hypothesized that SB3 might either contribute or to be involved in the genesis and/or maintenance of the hyperdynamic circulatory syndrome of cirrhosis.
The aim of the study was to investigate the relationships between SB3 and systemic and splanchnic hemodynamics. For this purpose, we analyzed the hemodynamic differences between transgenic mice expressing human SB3 and control animals, and how such differences were modified by the induction of liver fibrosis by chronic carbon tetrachloride (CCl4) treatment. In this study, we also evaluated liver and heart histopathology and investigated in vitro effects of human SB3 on mesenteric regulation.
2Materials and methods2.1Experimental animalsC57BL/6J mice transgenic (TG) for human SB3 (kindly provided by Professor G. Cassani, Technogen S.c.p.A, Piana di Monte Verna, CE, Italy) and C57BL/6J wild type (WT) control mice (Charles River Italia S.p.A, Calco, Lecco, Italy) were used as experimental model. Transgenic mice showed high SerpinB3 expression in the liver, evaluated by mRNA quantification and immunohistochemistry, while this serpin was barely detectable in the liver of control mice (Suppl. Figure 1 and Refs. [9,15]).
Moreover, 5 WT Wistar-Kyoto rats (male, 16 weeks old, body weight, 200–250g; Charles River Laboratories, Calco, Lecco, Italy) were used to analyze the effects of recombinant SB3 on mesenteric vascular reactivity. Mice and rats were kept under specific pathogen-free conditions and maintained with free access to pellet food and water at the Animal Care Facility of the Center of Experimental Surgery, University of Padua, Italy.
All institutional and national guidelines for the care and use of laboratory animals were followed. The experiments were carried out in accordance with the Italian legislation (D.L. 27/01/1992 116), which complies with the European Community guidelines (CEE Directive 86/609) for the care/use of experimental animals. All animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985). The experimental protocols were approved by the local Ethical committee and by the Italian Ministry of Health (ethics number: 202/2011-B). All measures were taken to minimize any pain or discomfort to the animals.
2.2Induction of liver fibrosisLiver fibrosis was induced by CCl4 (Sigma-Aldrich, Milan, Italy) in both WT and TG mice (males, aged 12–14 weeks old). CCl4 intoxication is a widely used model of experimental liver fibrosis [16], that induces necrosis and hepatocyte apoptosis with activation of hepatic stellate cell and tissue fibrosis [17].
Sixteen mice (8 TG and 8 WT) were inoculated intraperitoneally with CCl4 at a dose of 50μl/100g body weight, dissolved in olive oil (20%), twice a week for 10 weeks [18]. The control group (6 TG and 6 WT) received injections of olive oil vehicle twice a week for 10 weeks. All mice were sacrificed 24h after the last dose of treatment. After sacrifice, blood was obtained by cardiac puncture and the liver and spleen excised, weighed and divided into samples. At least two tissue samples were taken from the liver of each animal: the first one was formalin fixed and paraffin embedded for histological analysis (hematoxylin–eosin and Sirius red staining), while the other was immediately frozen in liquid nitrogen and stored at −80°C for molecular studies. For the evaluation of heart remodeling, 3 TG and 3 WT mice without CCl4 treatment were considered.
2.3Mouse liver histologyHematoxylin–eosin and Sirius red stainings were carried out according to standard protocols. Immunohistochemistry for SB3 and α-smooth muscle actin (α-SMA) was performed on paraffin-embedded sections of all liver specimens, obtained from treated and control mice using peroxidase block to eliminate endogenous peroxidase activity. After washing, sections (2μm thick) were incubated with anti-α-SMA (1:200 dilution; Sigma-Aldrich, Milan, Italy) and with monoclonal antibody against SerpinB3 (Santa Cruz Biotechnology, CA, USA; dilution 1:50) respectively. These antibodies were used diluted in phosphate-buffered saline supplemented with bovine serum albumin, overnight at 4°C in a humid environment, followed by incubation with peroxidase labeled polymer conjugated to secondary antibodies (HRP-labelled System; DAKO, Milan, Italy). The immunoreaction product was visualized by adding substrate-chromogen diaminobenzidine (DAB) solution, resulting with brownish coloration at antigen sites. Negative controls were performed by replacing the respective primary antibody by isotype and concentrations matched irrelevant antibody.
2.4Quantitative real-time PCR (SYBR green assay)The expression of SerpinB3 and Collagen 1 genes in the liver was assessed by real-time PCR using the following sets of primers: for SB3 sense primer 5′-GCAAATGCTCCAGAAGAAAG-3′, reverse primer 5′-CGAGGCAAAATGAAAAAGATG-3′. For Collagen 1 sense primer: 5′-GGGCAAGACAGTCATCGAAT-3′, antisense: 5′-GGTGGAGGGAGTTTACACGA-3′. The housekeeping gene mouse β-actin was analyzed in all amplification sets to assess the integrity of total RNA extracts and to define the relative transcript quantification of each gene of interest. The set of β-actin primers is: sense: 5′-AGAGCTACGAGCTGCCTGAC-3′ and antisense: 5′-GGATGCCACAGGACTCCA-3′. The relative expression was generated for each sample by calculating 2−ΔΔCt[19].
2.5Mouse heart histology and tissue analysisMacroscopic and microscopic analyses were performed on the WT and TG specimens. All samples were fixed in 10% formalin in 0.1mol/L (pH=7.4) phosphate buffer, dehydrated in a series of rising alcohol concentrations, and then embedded in paraffin for light microscopy. Four micrometer-thick sections were stained with hematoxylin and eosin and Heidenheim modified Azan-Mallory [20].
2.6Morphometric assessment of the heartThe following indices were calculated by computerized planimeter (Image Proplus) on formalin-fixed transverse section of the heart taken in the middle portion of interventricular septum:
- -
Left ventricular mass/right ventricular mass (LVM/RVM), index of hypertrophy.
- -
Left ventricular mass/left ventricular volume (LVM/LVV), index of dilatation of left ventricle.
- -
Right ventricular mass/right ventricular volume (RVM/RVV), index of dilatation of right ventricle.
These indices were calculated with a validated procedure currently adopted in our laboratory [20–22].
2.7Silver nitrate staining for AgNORTo assess cellular proliferation, Nucleolar Organizer Regions (AgNOR) were evaluated in sections of formalin-fixed paraffin-embedded hearth tissue samples, as previously described [23]. Briefly, Gelatin was dissolved in 1% aqueous formic acid at a concentration of 2%. This solution was mixed with 50% aqueous silver nitrate solution (1:2), and the final solution was poured over the tissue sections, which were incubated for 30min under dark room conditions at room temperature. They were then washed with deionized distilled water before counterstaining with Mayer's hematoxylin and dehydration by alcohols and xylene. Synthetic medium was used to mount the sections. The total number of AgNOR dots/nucleus at a 1000× magnification (oil immersion) in 200 representative nuclei were counted.
2.8Hemodynamics evaluationAfter anesthesia induced with 3% isoflurane and maintained with 1.5% isoflurane during constant monitoring of temperature, respiration rate, and ECG, all TG and WT mice, treated and untreated with CCl4, underwent to cardiac, hepatic, renal and splenic hemodynamics evaluation using the Vevo 2100 apparatus (Visualsonics, Toronto, Canada), a high-resolution in vivo micro-imaging system for small animals. The following parameters were measured: cardiac output (CO), portal vein (PV) diameter, portal vein blood velocity (PBV) and flow volume (PBF), hepatic, splenic and renal arterial resistance indices: resistance index (RI) and pulsatility index (PI). All these parameters were calculated as described elsewhere [24–26]. Mice underwent anesthesia, induced in a chamber for inhalation of isoflurane, before undergoing the sonographic examination. Mice were then put supine on a specific table, on which they were maintained anesthetized with isoflurane, while body temperature, heart and respiratory rates were monitored. Thorax and abdomen of mice were then shaved to allow an adequate ultrasonographic window. To eliminate inter-observer variability of Doppler results, all the measurements were recorded by a single operator, who was unaware of the treatment and the characteristics of the investigated mice.
2.9Isolated microvessel preparationSmall resistance mesenteric arteries of Wistar-Kyoto rats (diameter <500μm) were studied as previously described [26]. The no-flow model was chosen to avoid interference from shear stress.
2.10Evaluation of the direct effect of SB3 on mesenteric arteries preconstricted with phenylephrineThe preparation of isolated mesenteric arteries of Wistar-Kyoto rats [26] was used to evaluate the in vitro effect of recombinant SB3 on vascular diameter. The recombinant protein SB3 was prepared as previously described [27]. The direct effect of SB3 on mesenteric arteries was evaluated after preconstriction of the arteries with phenylephrine (PHE) (1μM). Recombinant SB3 was dissolved into physiological salt solution (PSS) to obtain a concentration of 10−7M. This concentration was empirically chosen, considering the vasoactive effect of an equimolar dose of another serpin, Angiotensin II [28]. SB3 was administered in the perfusion chamber (extraluminal application) and its effect was evaluated for at least 5min, measuring the change in arterial diameter with a video system [26].
2.11Evaluation of dose–response curves to phenylephrine and SB3 on mesenteric arteries, before and after inhibition of Angiotensin II type 1-receptors with irbesartanThe preparation of isolated mesenteric arteries of Wistar-Kyoto rats described elsewhere [26] was used to evaluate the effect of recombinant SB3 on phenylephrine (PHE) vasoconstriction. After the equilibration period, concentration–response curves to PHE at cumulative doses (10−8 to 10−4M) were evaluated. After a proper wash out period of at least 20min, mesenteric arteries were challenged again with increasing doses of PHE (10−8 to 10−4M) and SB3 (10−8 to 10−4M) simultaneously administered by extraluminal application, and concentration–response curves were re-evaluated.
The effect of SB3 after inhibition of Angiotensin II type 1-receptors with irbesartan was also analyzed. Irbesartan at the concentration of 10−5M was added to the solution, as previously described [29]. After a 20-min period of equilibration, mesenteric arteries were first challenged with PHE alone at increasing concentrations (10−8 to 10−4M) and concentration–response curves were recorded. Then, after a washout with PSS and irbesartan for at least 20min, the arteries were re-challenged with increasing doses of PHE (10−8 to 10−4M) and SB3 (10−8 to 10−4M) simultaneously administered by extraluminal application.
2.12Data analysisParameters of fibrosis and morphometric analysis on hearts were expressed as mean±SD or median±SEM and analyzed using the unpaired t-test, unpaired t-test with Welch's correction and non-parametric Mann–Whitney U test when appropriate. Hemodynamic data were expressed as mean±SD and analyzed with unpaired t-test. The effects of PHE and SB3 were evaluated as variations in the internal diameter of the vessels. Data were expressed as mean±SE. Two-way ANOVA was used to compare concentration–response curves from controls and treated groups. The null hypothesis was rejected at p<0.05.
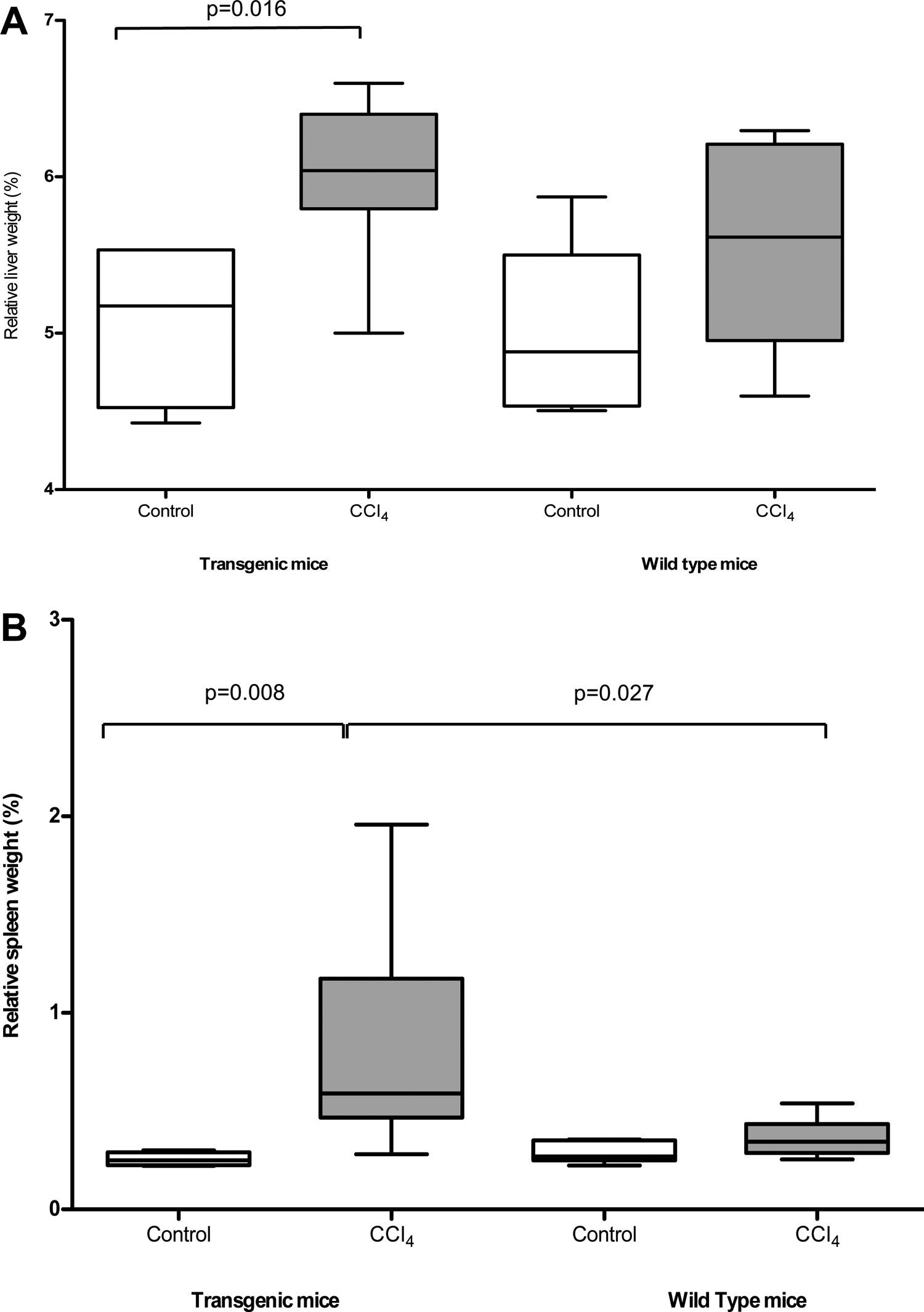
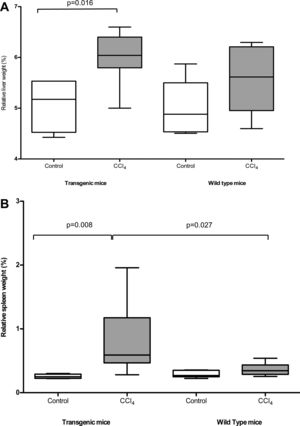
3Results3.1Pathological findings of the liverAfter CCl4 treatment, TG mice showed a significant increase of the relative volume of the liver and of the spleen, compared to untreated mice (liver/body weight ratio: 6.01±0.50% vs 5.55±0.63%, p=0.016; spleen/body weight ratio; 0.77±0.51% vs 0.28±0.06%, p=0.008), while WT mice did not show significant differences (Fig. 1A and B respectively). Accordingly, livers of TG vs WT mice treated with CCl4 showed an increased collagen synthesis/accumulation and an exacerbated myofibroblast activation (Suppl. Figure 2a and 2b), as resulted by data related to collagen 1 mRNA expression (Suppl. Figure 2c), confirming that overexpression of SB3 in TG mice is pro-fibrogenic in this model of chronic injury [10].
Relative weight of liver (panel A) and spleen (panel B) in TG and WT animals after olive oil (control) or CCl4 treatment. Results refer to 16 treated mice (8 TG and 8 WT) and to 12 untreated control mice (6 TG and 6 WT). The box indicates the lower and the upper quartile and the middle line indicates the median; bars indicate the range of values distribution (Mann–Whitney test).
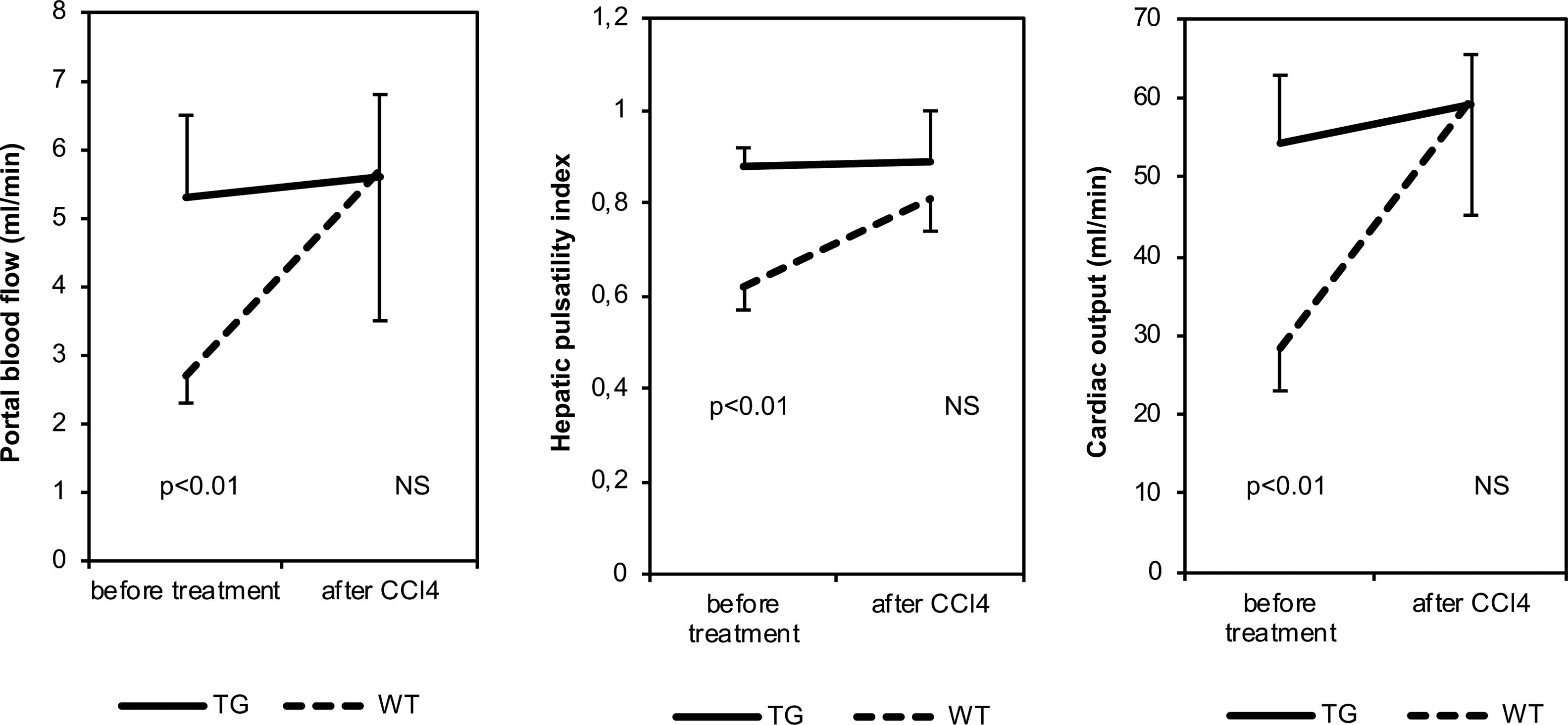
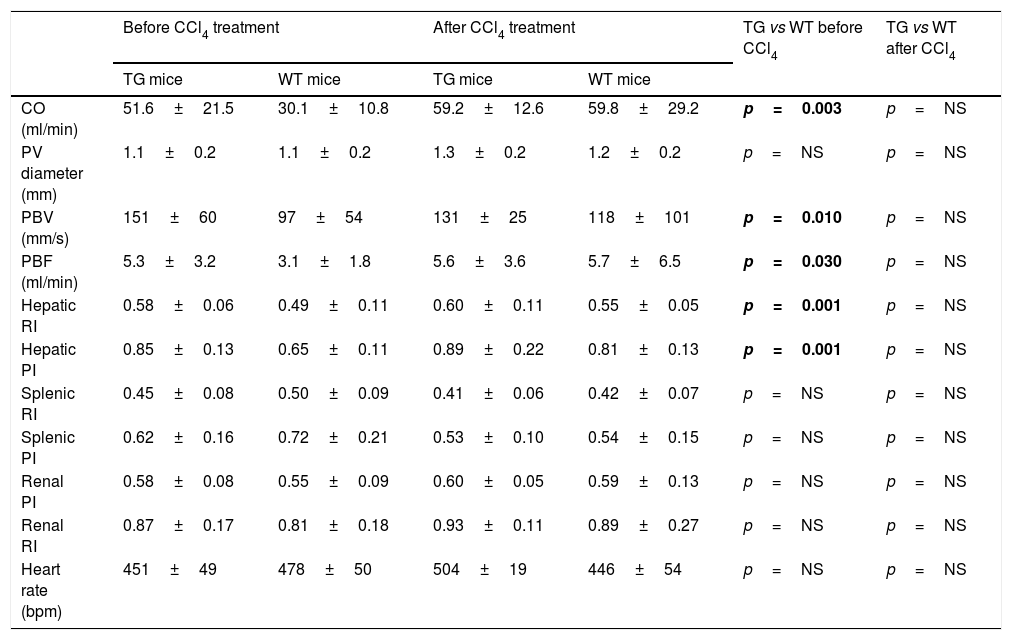
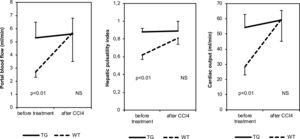
Before CCl4 treatment, CO, PBV, PBF, hepatic RI values and hepatic PI values were significantly higher in TG mice than in WT animals (Table 1 and Fig. 2). After CCl4 treatment, CO, PBV, PBF, hepatic RI and PI values were not modified in TG mice, while they increased in WT mice. These parameters were similar in all CCl4 treated animals. As far as heart rate, splenic and renal resistance indices are concerned, no significant difference was found among the investigated groups.
Hemodynamic parameters of TG and WT mice before and after treatment with CCl4.
| Before CCl4 treatment | After CCl4 treatment | TG vs WT before CCl4 | TG vs WT after CCl4 | |||
|---|---|---|---|---|---|---|
| TG mice | WT mice | TG mice | WT mice | |||
| CO (ml/min) | 51.6±21.5 | 30.1±10.8 | 59.2±12.6 | 59.8±29.2 | p=0.003 | p=NS |
| PV diameter (mm) | 1.1±0.2 | 1.1±0.2 | 1.3±0.2 | 1.2±0.2 | p=NS | p=NS |
| PBV (mm/s) | 151±60 | 97±54 | 131±25 | 118±101 | p=0.010 | p=NS |
| PBF (ml/min) | 5.3±3.2 | 3.1±1.8 | 5.6±3.6 | 5.7±6.5 | p=0.030 | p=NS |
| Hepatic RI | 0.58±0.06 | 0.49±0.11 | 0.60±0.11 | 0.55±0.05 | p=0.001 | p=NS |
| Hepatic PI | 0.85±0.13 | 0.65±0.11 | 0.89±0.22 | 0.81±0.13 | p=0.001 | p=NS |
| Splenic RI | 0.45±0.08 | 0.50±0.09 | 0.41±0.06 | 0.42±0.07 | p=NS | p=NS |
| Splenic PI | 0.62±0.16 | 0.72±0.21 | 0.53±0.10 | 0.54±0.15 | p=NS | p=NS |
| Renal PI | 0.58±0.08 | 0.55±0.09 | 0.60±0.05 | 0.59±0.13 | p=NS | p=NS |
| Renal RI | 0.87±0.17 | 0.81±0.18 | 0.93±0.11 | 0.89±0.27 | p=NS | p=NS |
| Heart rate (bpm) | 451±49 | 478±50 | 504±19 | 446±54 | p=NS | p=NS |
TG: transgenic for human SB3; WT: wild-type; CO: cardiac output; PV: portal vein; PBV: portal blood flow velocity; PBF: portal blood flow volume; RI: arterial resistance index; PI: arterial pulsatility index; bpm: beats per minute.
Bold values correspond to significant p values (p<0.05)
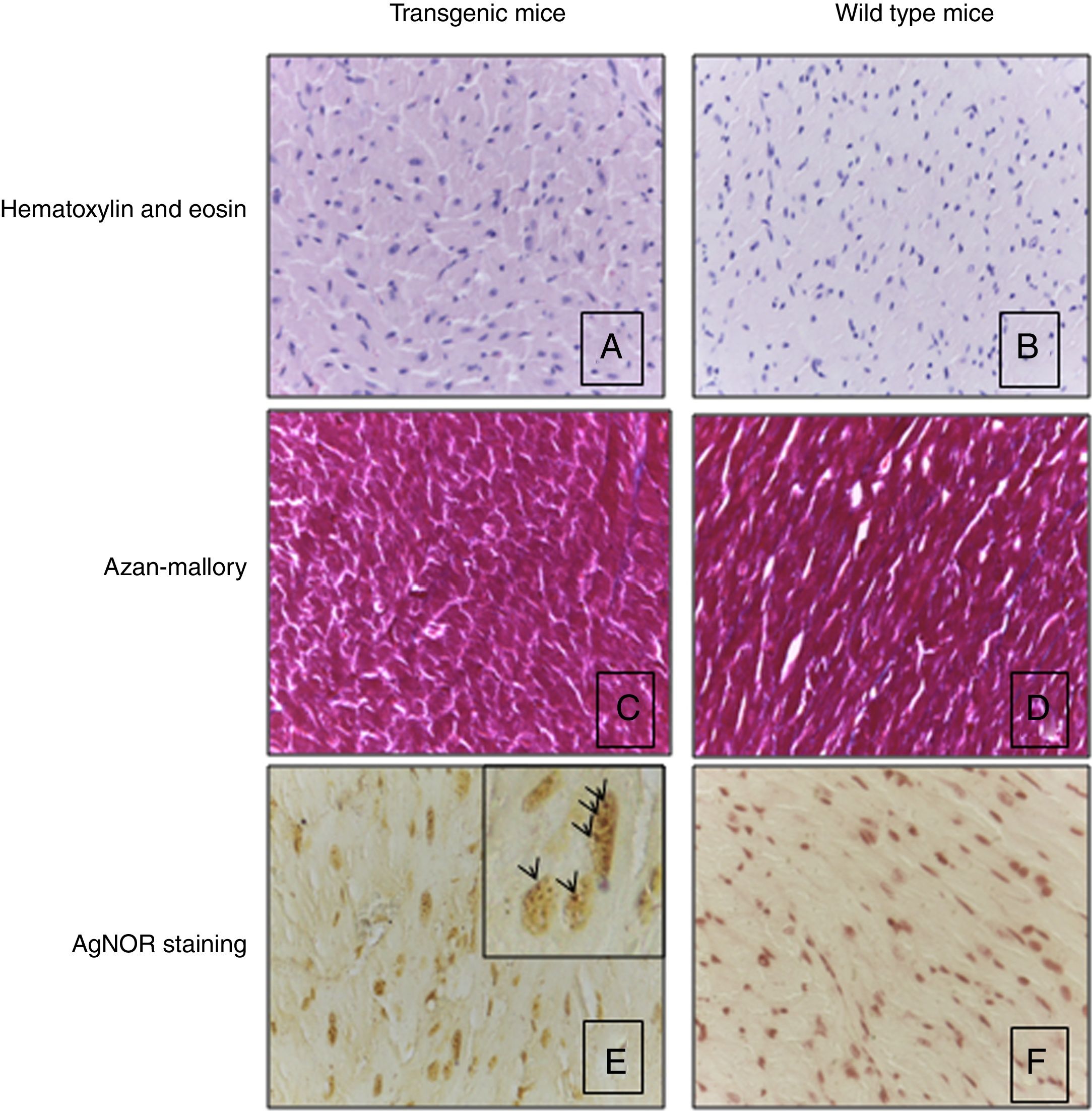
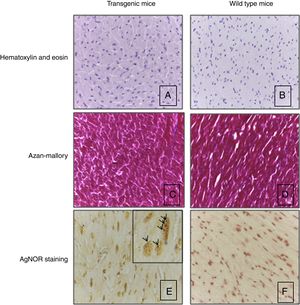
Heart histological evaluation of WT mice and TG mice did not show significant differences in terms of morphometric parameters, including capillary density (583.8±145.2n/mm2 and 516.2±49.5n/mm2 respectively), myocyte diameter (12.1±1.5 and 11.2±1.2μm), as reported in the representative Fig. 3A and B. Silver nitrate stain for AgNOR protein sites did not reveal a different number of proteins sites in wild type mice vs transgenic mice (2.74±0.71 and 3.37±0.74, respectively) (Fig. 3E and F).
Heart histology and tissue analysis. Hematoxylin and eosin (A, B) and Azan-Mallory stain (C, D) of transgenic mice showed the normal orientation, diameter, nuclear shape and no increase of interstitial fibrosis in respect of wild type mice. Panels A–D: original magnification 40×. AgNOR staining was the same in transgenic and wild type mice (E, F). Note intranuclear black dots in the nucleus of cardiomyocytes that indicates the presence of AgNOR sites. Panels E, F: original magnification 63×. Results refer to 6 untreated mice (3 TG and 3 WT).
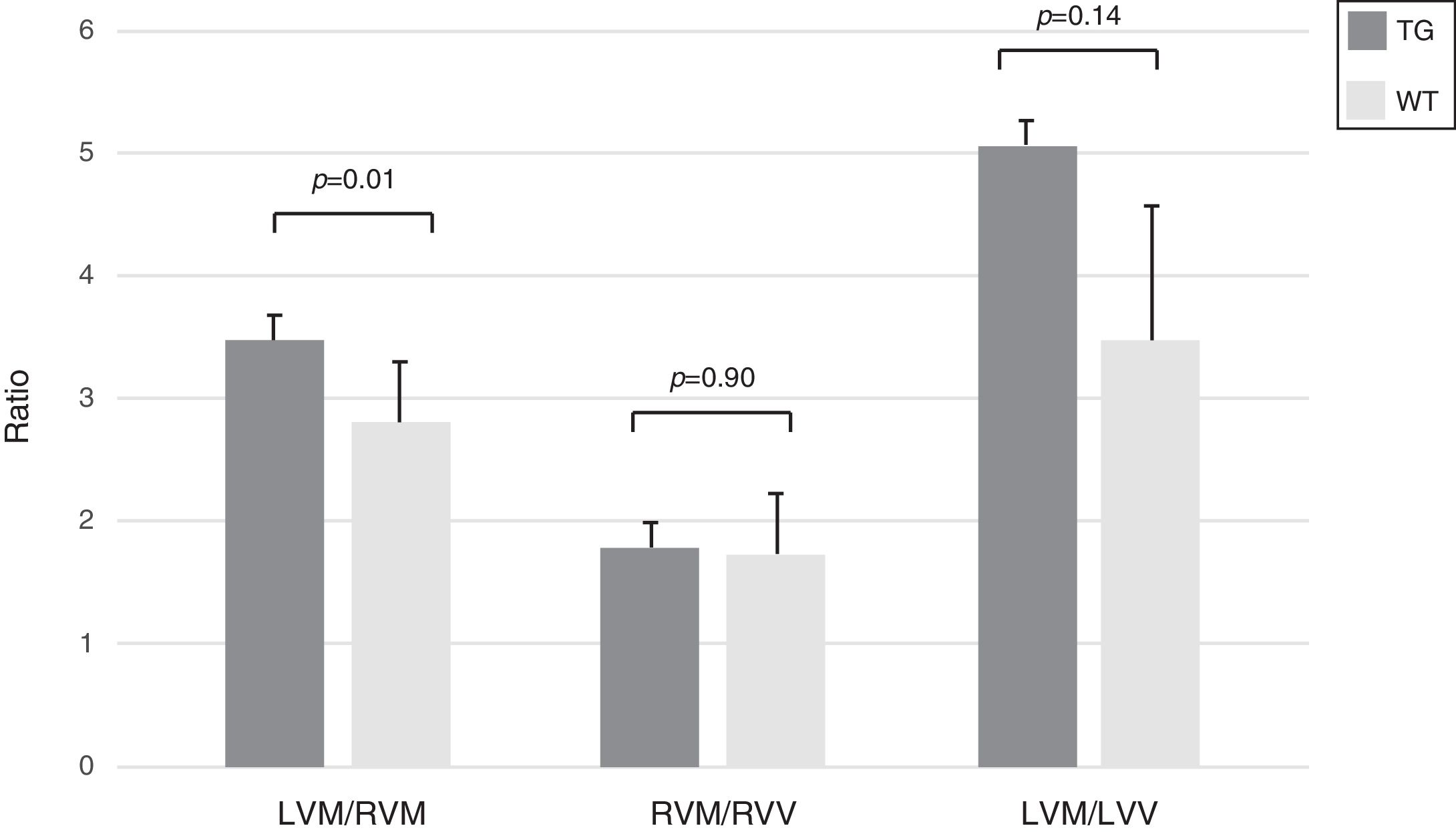
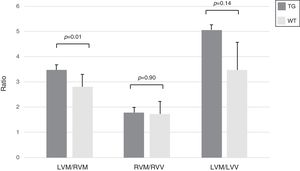
In transgenic mice the index of hypertrophy (LVM/RVM ratio) was significantly higher compared to wild type mice (p=0.01) (Fig. 4).
Morphometric assessment of the heart in WT and TG mice. The index LVM/RVM ratio is significantly higher in TG mice compared to the controls. Results refer to 6 untreated mice (3 TG and 3 WT). Columns are means±SD. LVM: left ventricular mass; RVM: right ventricular mass; LVV: left ventricular volume; RVV: right ventricular volume.
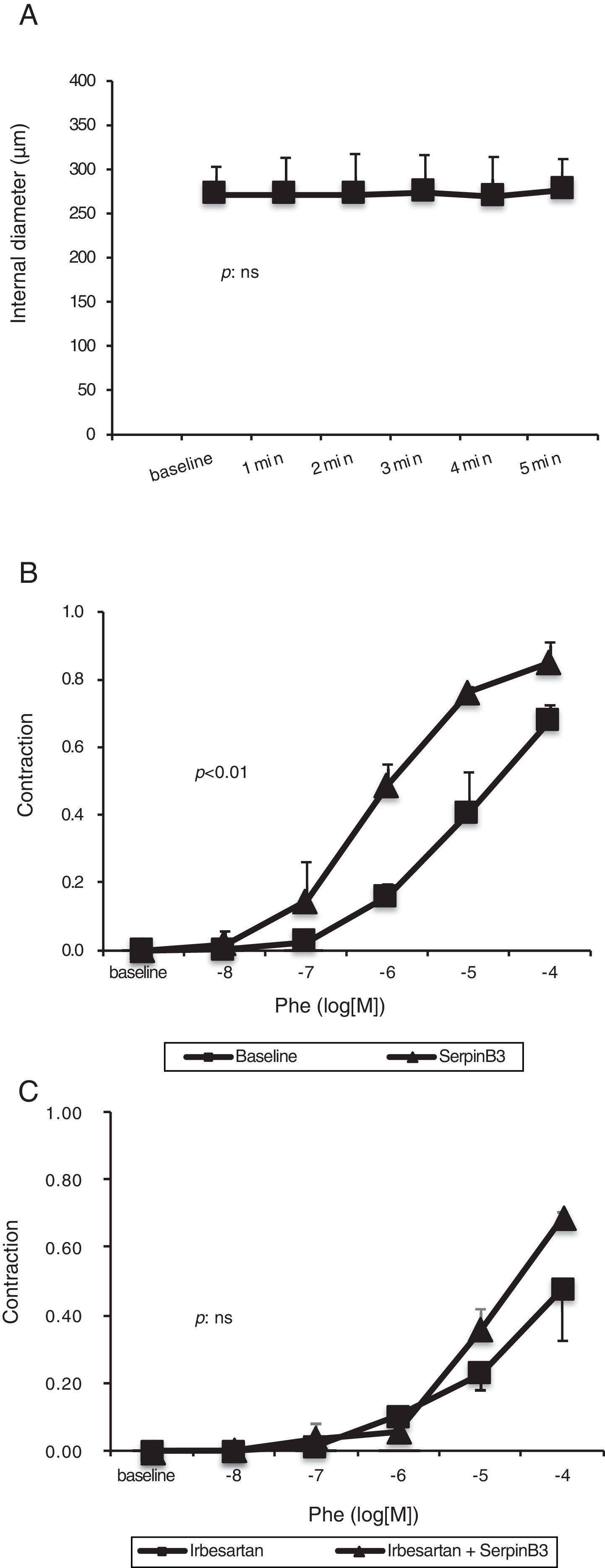
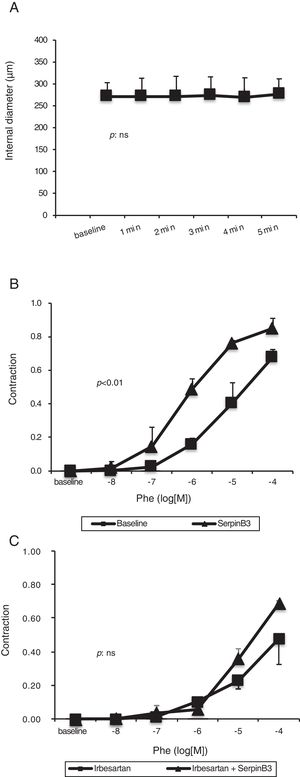
Mesenteric arteries vasoconstriction obtained after PHE (10−6M) challenge was not further influenced by the extraluminal administration of recombinant SB3 (Fig. 5A).
Panel A: effect of SB3 (10−7M) on small resistance mesenteric arteries (isolated from to 5 WT Wistar-Kyoto rats) preconstricted with phenylephrine (10−6M). No significant variation in arterial diameter was observed. Panel B: concentration–response curve to phenylephrine (PHE) obtained in small resistance mesenteric arteries. The administration of SB3 increased the sensitivity to PHE of the arteries (two-way ANOVA: p<0.01). Panel C: concentration–response curve to PHE obtained in small resistance mesenteric arteries incubated with irbesartan (10−5M) to inhibit Angiotensin II type 1-receptors. After pre-incubation with irbesartan, the administration of SB3 did not modify the sensitivity to PHE of the arteries (two-way ANOVA: p: NS). Estimated of variance are±SD.
This molecule alone had no acute hemodynamic effect on mesenteric arteries in vitro, however it increased the sensitivity to PHE of small resistance mesenteric arteries (two-way ANOVA: p<0.01) (Fig. 5B).
Arterial sensitivity to PHE was decreased by pre-treatment with irbesartan (two-way ANOVA: p<0.01) but was not further modified by the administration of SB3 (two-way ANOVA: p=NS) (Fig. 5C).
4DiscussionThe present study demonstrates that SB3 has a vasoactive effect on mesenteric circulation and may participate in the genesis of the hyperdynamic circulatory syndrome of liver cirrhosis, besides being involved in the progression of hepatic fibrogenesis [10].
The novelty of our results resides on the fact that in our experimental model, despite CCl4 treated TG mice showed more advanced morphological and molecular features of hepatic fibrosis, compared to controls, SB3 has demonstrated a vasoactive effect on splanchnic circulation. A direct participation of this molecule in the pathophysiology of the hyperdynamic circulatory syndrome of cirrhosis, which maintains and worsens portal hypertension, may therefore be hypothesized.
Our results on untreated mice have shown that, compared to WT animals, TG mice have significantly higher values not only of portal vein blood velocity and flow volume, but also of cardiac output. In addition, in untreated TG mice, an increase in hepatic artery resistance index was detected and this finding is in agreement with the so called “hepatic arterial buffer response theory”. According to this theory, hepatic arterial resistance physiologically increases when PBF increases, to maintain total hepatic blood flow constant [30]. On the other hand, a direct effect of SB3 on intrahepatic arterial resistance cannot be ruled out. One limitation of this work is the lack of murine urine analysis to confirm the presence of an higher sodium retention in TG mice compared to WT mice. However different hemodynamic parameters have been evaluated in these mice by an high-resolution in vivo micro-imaging system, and no conflicting results have been found.
Both cardiac and splanchnic hemodynamic modifications described above are commonly found in liver cirrhosis, supporting the fact that our murine TG model for SB3 presents hemodynamic alteration typical of cirrhosis in the absence of liver structural subversion. Hemodynamic differences between TG and WT mice were abolished after CCl4 treatment, when also WT mice developed the hyperdynamic circulatory syndrome in a contest of advanced fibrosis deposition.
In line with the presented findings, morphometric analysis on heart tissue samples showed an increased cardiac hypertrophy in TG mice, possibly linked to their hyperdynamic circulation, while capillary density, numbers of myocytes or proliferation index were not significantly increased.
The study of vascular reactivity on Wistar-Kyoto rats showed that in vitro recombinant SB3 has no direct hemodynamic effect on mesenteric arteries whereas its acute administration modifies the sensitivity of the artery to alpha-adrenergic stimuli. Consistently with the data above discussed, we can suppose that this effect might be mediated by the renin–angiotensin–aldosterone system (RAAS), particularly by Angiotensin II type 1-receptors. This hypothesis, that needs to be further explored in future studies, has been confirmed and supported by data from experiments in which SB3-mediated increase in mesenteric vasoconstriction, was blocked by the administration of irbesartan, a specific inhibitor of Angiotensin II type 1-receptors.
In conclusion, SB3 seems to participate to the genesis of the hyperdynamic circulatory syndrome, possibly mediated by angiotensin receptors, beside its involvement in the progression of liver fibrosis, leading to the mechanical increase in intrahepatic portal resistance of cirrhosis.AbbreviationsCCl4
carbon tetrachloride
SB3SerpinB3
TGF-β1Transforming Growth Factor-β1
IL-6interleukin-6
TGtransgenic
WTwild type
COcardiac output
PVportal vein diameter
PBVportal vein blood velocity
PBFportal blood flow
α-SMAα-smooth muscle actin
RIresistance index
PIpulsatility index
PHEphenylephrine
LVMleft ventricular mass
RVMright ventricular mass
LVVleft ventricular volume
RVVright ventricular volume
PSSphysiological salt solution
RAASrenin–angiotensin–aldosterone system
NORsNucleolar Organizer Regions
AgNORsilver-stained Nucleolar Organizer Regions
Financial supportThis work was supported in part by National Ministry of Education, University and Research [FIRB Project Prot. RBLA03S4SP_005] and by the University of Padova [Project No. CPDA110795].
Conflict of interestNone.