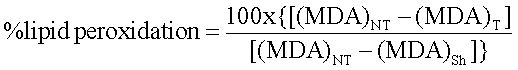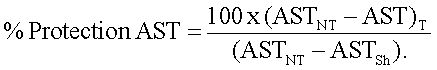Introduction. Effective prevention strategies require specific actions during the different phases of ischemia-reperfusion (I-R) injury. The objective of our study is to evaluate the effect of aqueous extract of Hypericum humifusum leaves (HHL) on liver I-R model in Rat.
Material and methods. Animals were subjected to 90 min of hepatic ischemia followed by reperfusion (120 min). HHL extract (25 mg/mL/kg) was injected 15 min before reperfusion. To evaluate the effect of HHL extract on I-R, we have monitored transaminases levels, Malondialdehyde (MDA) concentration, histological lesions (apoptosis and necrosis) and compared the results to a reference oxidant vitamin E.
Results. The determination of total phenol extracts of HHL was 59.91 ± 0.35 mg of Gallic Acid/g dry plant material with higher antioxidant activity (91.73% ± 1.67) compared to vitamin E (87.42%). Using aqueous extract of HHL, we noted a significant decrease of AST and ALT [1129 UI (585/1995) and 768 UI (335/1375)] compared to no-treated group [5,585.5 UI (5,035/12,070) and 8,099.5 UI (5,040/12,326)] as a decrease in MDA content [85.7% protection (50.9/91.5)]. HHL extract reduce the damage induced by I-R of 48.7% (27/48.7) and 96.1% (95.7/96.5) for necrosis and apoptosis lesions respectively.
Conclusion. HHL aqueous extract have potential to protect liver from the damage effect induced by I-R better than vitamin E solution.
Liver ischemia-reperfusion (I-R) injury involves pathophysiological disturbances leading to systemic and hepatic damage after hepatic trauma, circulatory shock or transplantation.1 Hepatic I-R injury is a result of multifactorial complex mechanisms including reactive oxygen species (ROS) generation to cause liver cell injury and death. The degree of I-R injury depends on the severity of ischemic insult.2 The increased release of ROS during I-R injury results in depletion of endogenous antioxidants. In this situation, the cells require exogenous antioxidant like plant extract to protect them from damage induced by ROS.3
Although substantial progress has been made for elucidation of mechanisms of I-R injury, there is still a need to better understand its pathophysiology and treatments mechanisms. During several years, many studies have been conducted on medicinal plants as alternative treatments in I-R and they demonstrate beneficial effect on some diseases.4,5 In fact, many plant extracts and their components like flavonoids and phenolic compounds have been shown to exert biological functions.6,7
The genus Hypericum (Hypericaceae) is represented by more than 458 species (trees, shrubs and herbs) subdivided into 36 sections. Species are distributed mainly in temperate regions of the Northern hemisphere and tropical high elevation habitats.8,9 The genus Hypericum is well known by its therapeutic effects. It has been used traditionally as antiinflammatory, sedative, analgesic, diuretic and antimalarial. It has also been used for the treatment of several illness such as trauma, burns, rheumatism, hemorrhoids, neuralgia, gastroenteritis, snakebite, ulcers, contusions, sprains, hysteria, bedwetting, and depression.10
H. perforatum is the most studied taxon and the only used in official medicine especially for antidepressive and antiviral activities.9,11 The other species remain not well known. H. humifusum L is found mainly in wet habitats in the Northand the Center of Europe, Argentina and several Mediterranean regions. In Tunisia’ the species grows widely in the north and the center of the country on sandy soils under a rainfall ranging from 350 to 992 mm/year.12
To our knowledge, in liver injury, few reports demonstrate the protective effects of Hypericum perforatum leaves extract and no study reported the effect of Hypericum humifusum in I-R injury.
The objective of this study was to determine the acute effects of extract plant of Hypericum humifusum leaves (HHL) in I-R liver injury on an in vivo rat model.
Material and MethodsPlant material and extraction procedurePlant material identification was performed by Pr Mohamed Boussaid (Botanic professor). Hypericum humifusum has been collected in the region of Mornaguia (south-west of the capital Tunis) in September.
Four grams of HHL was reduced to a fine powder in the presence of liquid nitrogen and was mixed with methanol (100 mL, 18 h). After maceration, the methanolic extract was centrifuged (4,000 g, 15 min), the combined filtrates were evaporated and dissolved in NaCl 9%o (100 mL). Finally, a centrifugation (15,000 g, 15 min) was performed, and the supernatant was conserved in sterile tubes (4 °C, at obscurity).
Determination of total phenolic contentWe have used the Folin-Ciocalteu (FC) colorimetric method to estimate total phenolic content. HHL extract (50 µL) was diluted (10 folds) with NaCl 9%o and mixed with FC reagent (750 µL). After dilution with distilled water (10 folds), the mixture was protected from light and incubated at 37 °C for 5 min. 750 µL of Na2CO3 (60 g/L) solution was added to the mixture. Then, the absorbance was measured at 725 nm after a rest of 90 min. A standard range was prepared in the same conditions as the samples using different concentrations of Gallic Acid.13
2,2-diphenyl-1-picrylhydrazyl radical scavenging assayDue to its unpaired electron, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical gives a strong absorption band at 517 nm (deep violet color).
Twenty-five µL of HHL extract (or vitamin E solution) was mixed with DPPH radical (1 mL), the final concentration was 0.04 mM. Mixtures were protected from light and incubated for 30 min. 1 mL of DPPH (0.5 mM) diluted in 4 mL of NaCl 9% was used as control. The antioxidant activity was expressed in percentage of the inactivated DPPH radical according to the following formula: DPPH radical inactivation = 100 (A0 - As) / A0.
A0: absorbance of the control at 517 nm (containing all reagents except the test compound);
As: absorbance of the sample at 517 nm.
Animal experimentationAll animal procedures used in this study are in strict accordance with the “Guide for the Care and Use of Laboratory Animals” prepared by the National Academy of Sciences and published by the National Institutes of Health (NIH publication 86-23 revised 1985) USA.
In this experimental study, adult male Wistar rats (264 ± 50 g) were provided by Pasteur Institute of Tunis, Tunisia and housed in the Department of Animal Experimentation of the Faculty of Medicine of Tunis, Tunisia.
Animals were subdivided in four groups (n = five for each group) as follows:
- •
Sham group: liver rats without ischemia or reperfusion,
- •
No-treated group: ischemia = 90 min and reperfusion = 2 h,
- •
Treated vitamin E group: ischemia = 90 min and reperfusion = 2 h with injection of vitamin E (20 mg/ kg) in the femoral vein. Vitamin E was diluted in DMSO (Dimethyl Sulfoxide).
- •
Treated HHL group: ischemia = 90 min and reperfusion = 2 h with injection of Hypericum humifusum leaves extract (25 mg/kg) in the femoral vein. HHL was diluted in NaCl 9%.
The technique described by Nauta, et al. for normothermic liver ischemia was used.13,15 The surgical procedure was performed under general anesthesia. Hepatic normothermic ischemia was induced and maintained for 90 min by hilum clamping of the hepatic pedicles of segments I to V after liver ligaments section, the blood supply by the portal pedicles of segments VI and VII was not interrupted in order to avoid vascular congestion of the alimentary tract. During the period of ischemia, 0.5 mL of saline solution was given through the dorsal vein of the penis every 30 min to maintain hemodynamic stability and to replace losses due to portal stasis.
HHL extract or vitamin E solution was injected in the femoral vein 15 min before reperfusion.13,16 After 120 min of reperfusion (established by removing clamps), animals were sacrificed. Blood samples were collected for the measurement of transaminases levels. Liver lobes suffering from ischemic injury were immediately removed for mitochondria extraction (malondialdehyde (MDA) monitorage) and for histological analysis (liver was preserved in formol 10%).
MDA analysisMDA levels in the samples were determined to estimate oxidative damage of the membrane lipid. The Thiobarbituric acid (TBA) method was used to quantify MDA levels and estimate the lipid peroxidation in the liver, measured as TBA-reactive substances.
Hepatic mitochondrial (1.25 mg/mL) was incubated at 37 °C during 40 min. Trichloroacetic acid (3%) was added to stop the peroxidation reaction and the mixture was centrifuged (3,000 g, 15 min, 20 °C). The supernatant was added to TBA 1%, followed by an incubation during 30 min at 95 °C. The samples were then cooled, and their absorbances were measured using NaCl 0.9%o and TBA (v/v) as an external standard solution by spectrophotometric method at 530 nm.
Biochemical assayIn order to evaluate hepatic injury, liver enzymes alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were monitored by an automated analyzer (COBAS Integra800) using enzyme multiplied immunoassay technique (EMIT).
Results were expressed in percentage (%) of protection of AST and ALT using the following formula:
The same equation was used to determine the percentage of protection of ALT.
HistopathologyTissues were embedded in paraffin and sections of 4-5 mm were cut and stained with hematoxylin & eosin (H&E) and Trichrome of Masson (TM). TM associated three colors: nuclear color (hematoxylin), plasmatic color and blue or green color (reticuline fibers). TM allows the quantification of cells apoptosis and the determination of necrosis lesions areas.
Anatomopathologic study was focused on the qualification of the inflammatory infiltrate, the necrosis and the apoptosis cells by photonic microscope.
The percentage of apoptotic area was estimated by random evaluation of 10 high power fields (HPF) of sections stained with H&E and TM. The apoptotic and the non-apoptotic areas were mapped, thus the percentage of apoptotic area was estimated.
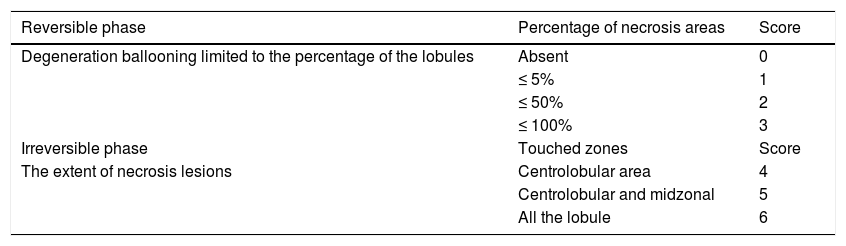
We evaluate semi quantitatively the necrotic areas with an established score chosen from 0 to 6 according to the stage, the topography and the severity of the lesion. Liver lobules are the structural units of the liver. Liver lobule is based on the direction of the blood flow from the portal space to drain in the central vein. Cells in the hepatic lobule are subdivided into three zones. Zone 1 (periportal zone) is closely related the portal canal, whereas zone 3 (centrolobular zone) is closer to the central vein and is the most distant from the oxygenated arterial blood supply. Zone 2 (midzonal) is intermediate between zone1 and zone 3 (Table 1).
Reversible and irreversible phases of necrosis.
| Reversible phase | Percentage of necrosis areas | Score |
|---|---|---|
| Degeneration ballooning limited to the percentage of the lobules | Absent | 0 |
| ≤ 5% | 1 | |
| ≤ 50% | 2 | |
| ≤ 100% | 3 | |
| Irreversible phase | Touched zones | Score |
| The extent of necrosis lesions | Centrolobular area | 4 |
| Centrolobular and midzonal | 5 | |
| All the lobule | 6 |
Results of in vitro study are given as means ± standard deviation (SD). Data of in vivo study are expressed in medians, minimum and maximum (min/max).
Statistical significance between groups were determinate by using Mann Whitney test (non-parametric assays). Values of P < 0.05 and P < 0.01 were considered statistically significant and highly significant, respectively.
ResultsPolyphenolTotal phenols determined by the FC method for aqueous extract of Hypericum humifusum leaves was 59.91 ± 0.35 mg of Gallic Acid /g dry plant material.
DPPHQuantitative analysis showed that HHL extract have higher DPPH radical scavenging property comparing to vitamin E solution (91.73% ± 1.67 vs. 87.42% ± 4.34).
Lipid peroxidation inhibition- •
In vitro. The antioxidant properties of HHL extract and vitamin E solution were cheeked from isolated liver mitochondria of control animals. Lipid peroxidation was induced by the addition of mixture of Fe2+/ Fe3+. HHL inhibited lipid peroxidation higher than vitamin E solution (100% vs. 60.62 ± 0.82%).
- •
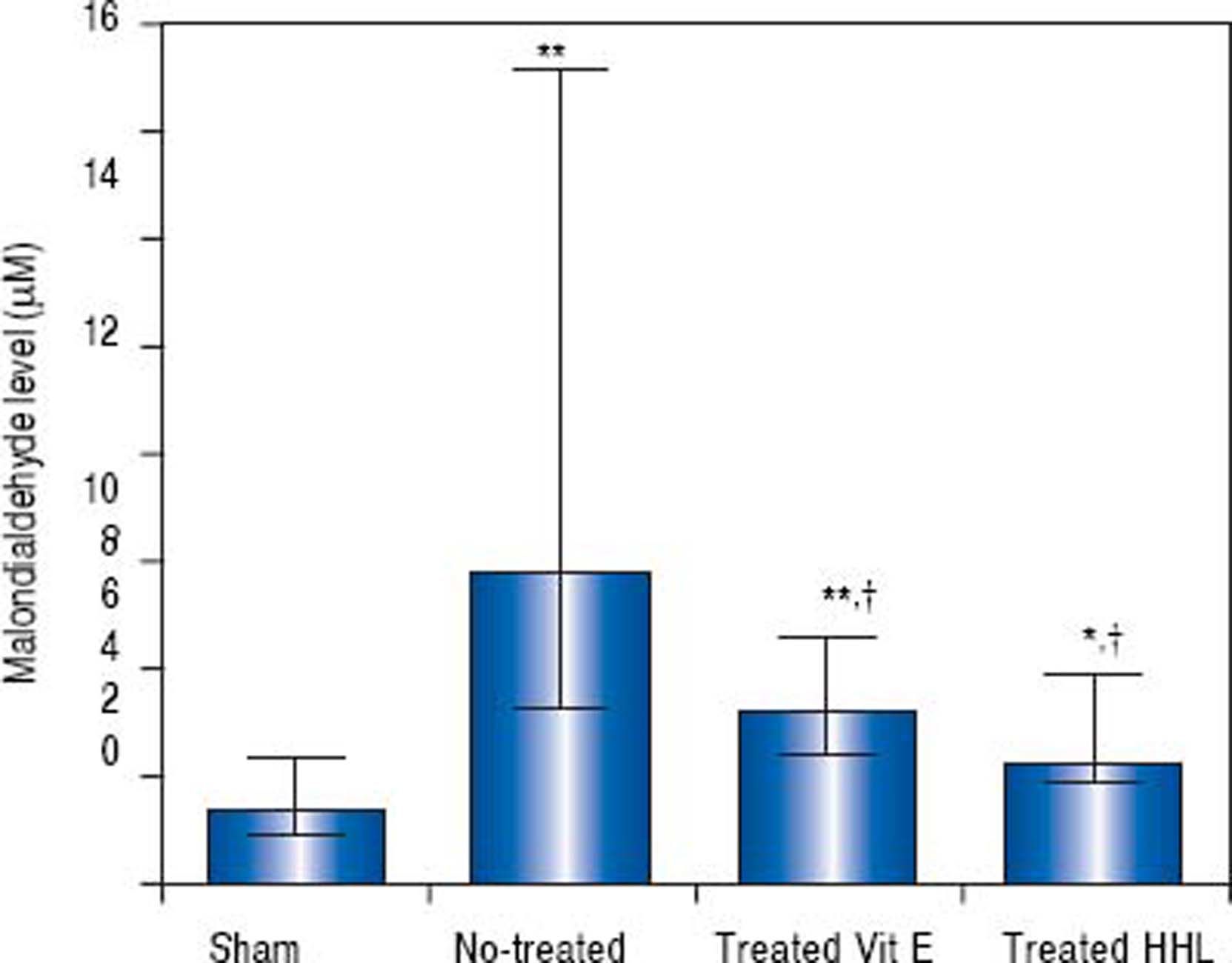
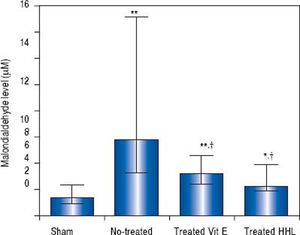
In vivostudy. The MDA content as an index of lipid peroxidation was significantly higher (P = 0.0001) after I-R in no-treated group [5.83 µM (3.3/15.1)] than in sham group [1.41 (0.9/2.34)].
In treated groups (with HHL extract or vitamin E solution), MDA decreased significantly (2.2 (1.92/3.97) and 3.2 µM (2.4/4.6) respectively) compared to no treated group (P = 0.024 and P = 0.017, respectively) (Figure 1).
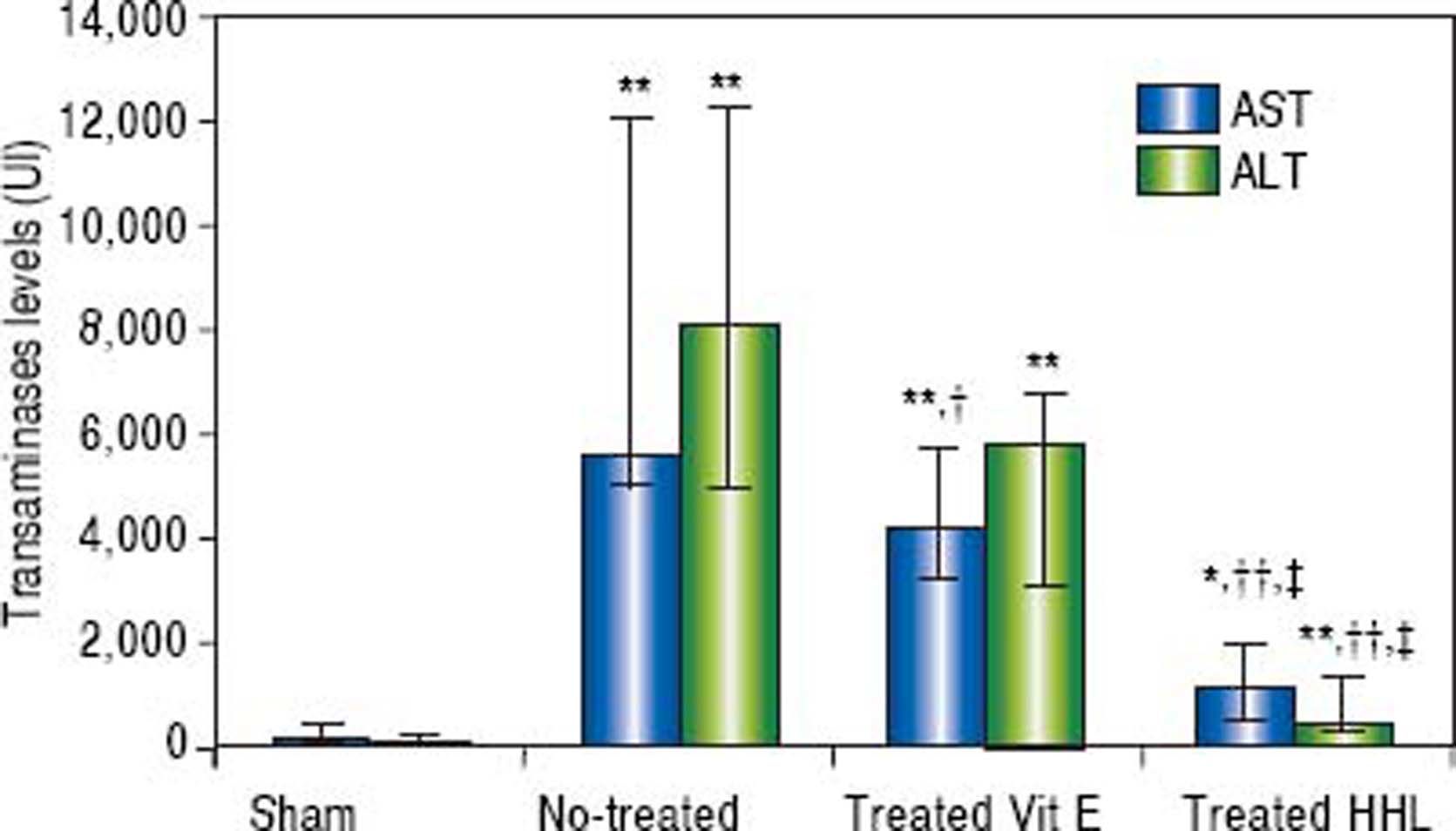
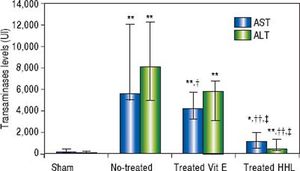
Serum transaminases levelsThe ALT and AST serum levels of the sham group were 63 UI (45/263) and 133 UI (106/479), respectively. A highly significant increase in serum ALT and AST levels (P = 0.001 and P = 0.0009, respectively) was observed after I-R in no-treated group compared to sham group [8,099.5 UI (5,040/12,326) and 5,585.5 UI (5,035 / 12,070), respectively].
A highly significant decrease of ALT and AST levels [768 UI (335/1,375) and 1,129 UI (585/1995), respectively] was observed after treatment with HHL extract compared with no-treated group (P = 0.004 and P = 0.002, respectively).
Treatment with vitamin E decreased significantly AST serum level 4,202 UI (3,286/5,783) compared to no-treated group (P = 0.023). For the ALT serum levels, there are no significant difference between no-treated group and treated vitamin E group [5,812 UI (3,093/6,746) (P = 0.09 > 0.05] (Figure 2).
Transaminases levels for each group after liver ischemia-reperfusion. Transaminases was measured in collected blood sample. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST). Values are expressed as medians (min-max). P < 0.01 ** vs. Sham, †† vs. no-treated, P < 0.05 *vs. Sham, †vs. no-treated, ‡vs. treated vitamin E.
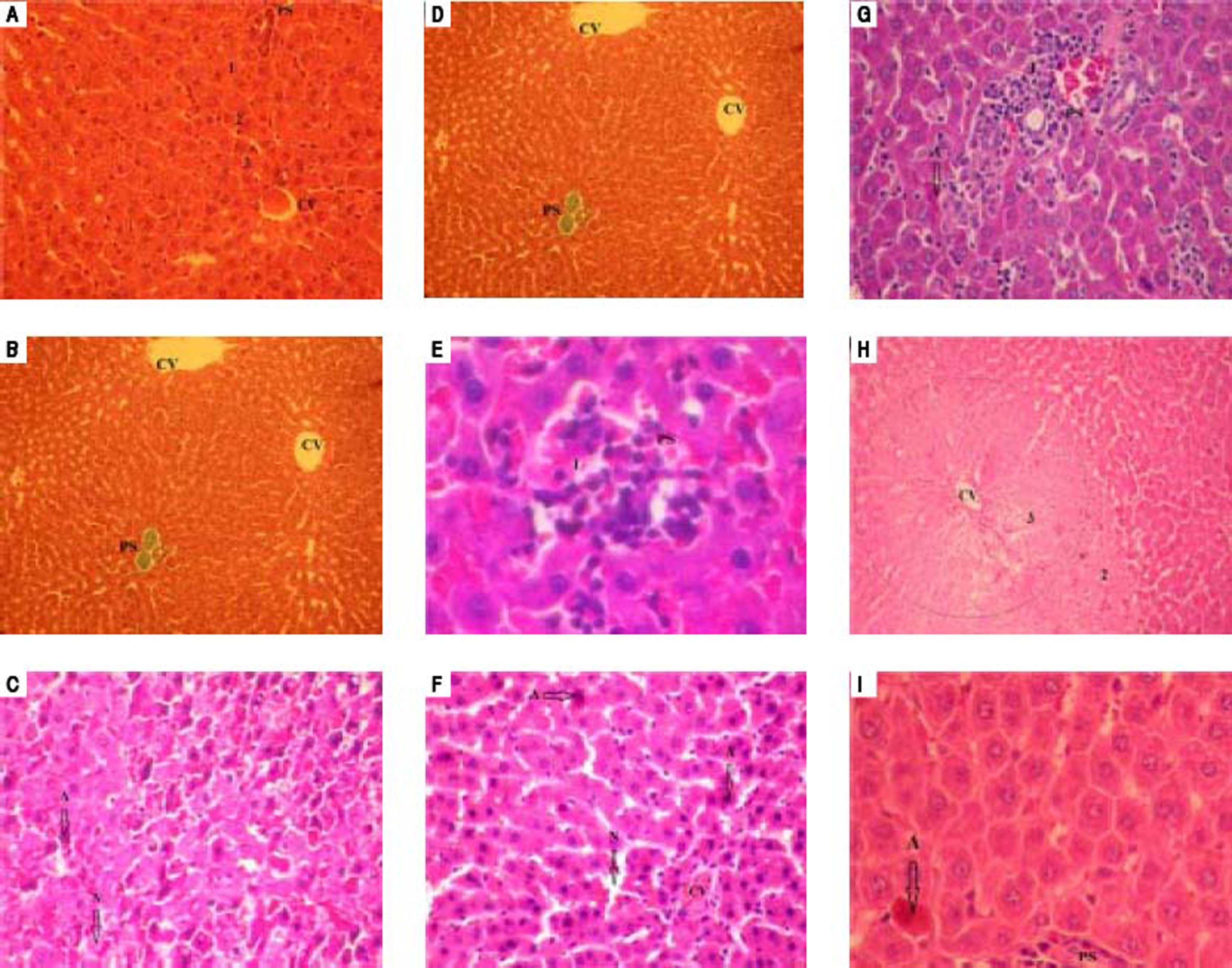
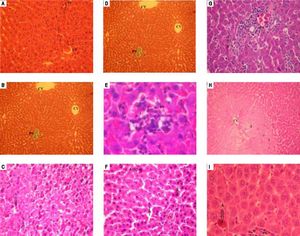
Histological features of normal liver tissue were observed in liver tissues obtained from sham group (Figures 3A and 3B). Histological examination of liver after 90 min of ischemia and 120 min of reperfusion revealed expected and characteristic pathological in the three zones (Figures 3C-3E).
Similar histology for vitamin E group and no-treated group was observed. The section of liver obtained demonstrate features of severe damage (irreversible necrosis and apoptosis cells) in the centrolobular and midzonal lobules (zones 2 and 3) with infiltration of inflammatory cells (Figures 3F and 3G).
Treatment with HHL aqueous extract induce persistence of reversible necrosis phase in zones 2 and 3 (Figure 3H), and a decrease of irreversible necrosis areas and apoptotic cells without inflammatory infiltrate (Figure 3I).
Histopathologic appearances of liver tissues among groups after ischemia-reperfusion phases. PS: portal space. CV: central vein. A: apoptosis. N: necrosis. I: inflammatory infiltrate. 1: zone 1; 2: zone 2; 3: zone 3. Normal appearance of liver in the Sham group (A) (H&E x 40) (B) (TM x 10). In the notreated group, liver is almost destroyed in the specimens from rat. Liver section shows necrosis areas and apoptosis cells was marked with intensity eosinophilic cytoplasm (C) (H&E x 40), (D) (TM x 40). (E) An important infiltration of inflammatory cells at portal canal (H&E x 400). From vitamin E group, (F) liver section shows necrosis and apoptosis cells (H&E x 40) (G) with infiltration of inflammatory cells at portal canal (H&E x 40). (H) Liver section of HHL group shows persistence of reversible necrosis phase (H&E x 10) (I) with decrease of apoptotic cells (H&E x 400). All histologic evaluation was done in a double-blinded fashion.
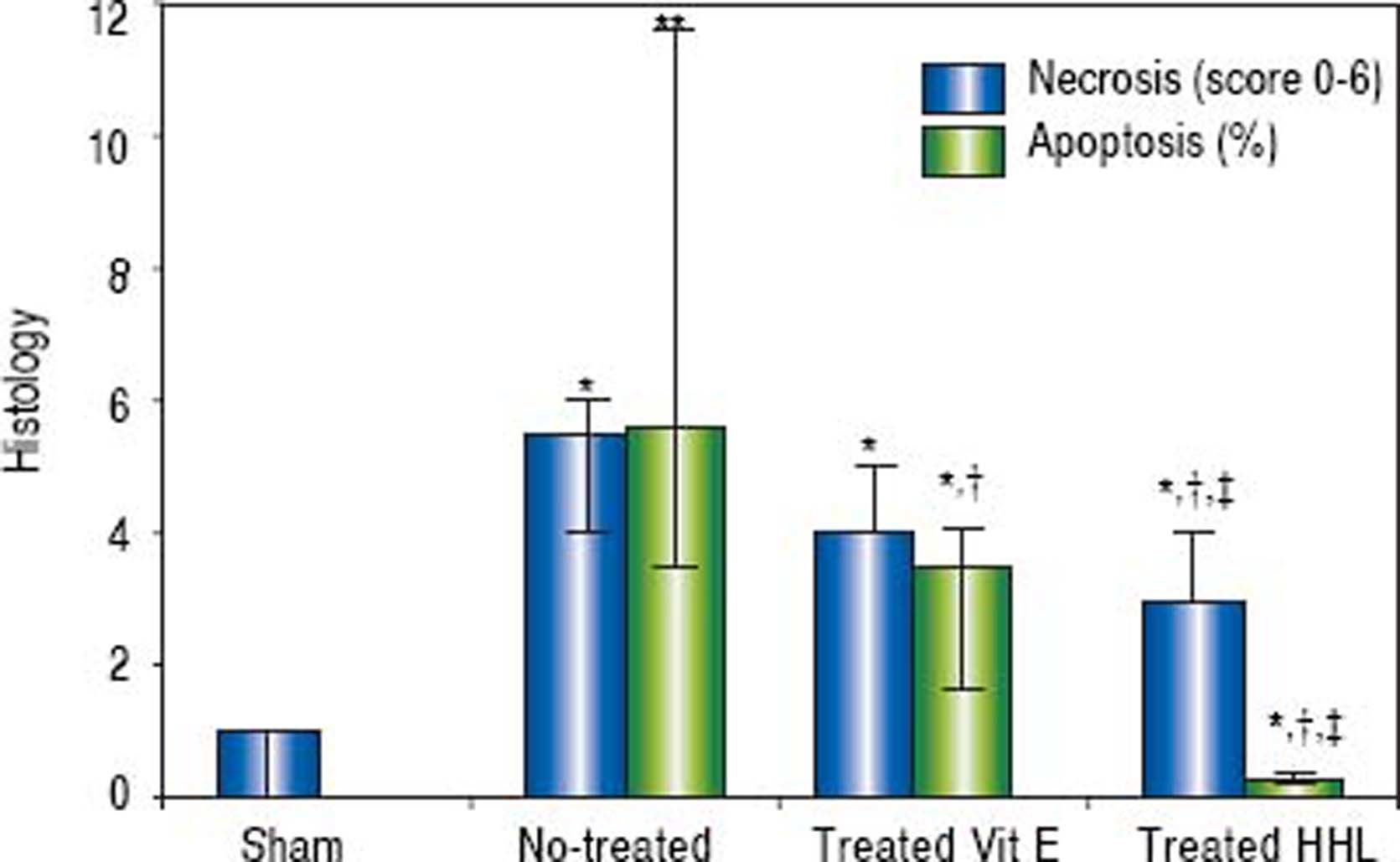
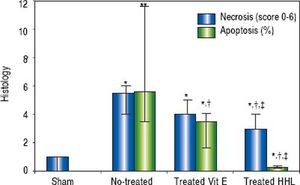
The semi quantitative evaluation of necrotic areas (score 0 to 6) was 1 (0/1) in the Sham group and it increased significantly to 5.5 (4/6) in no-treated group (P = 0.02). After treatment with HHL extract, necrotic areas decreased to 3 (3/4) compared to no-treated group and vitamin E treated group [3 (4/5)] (Figure 4).
Quantification of apoptosis and necrosis lesions for each group after liver ischemia-reperfusion. Score (0 to 6) according to the stage, the topography and the severity of necrosis lesion. Percentage of apoptotic area estimation by random evaluation of 10 HPF. Values are expressed as medians (min/max). P < 0.01 **vs. Sham.
The rate of apoptotic areas increased highly significant after I-R periods to 5.62% (3.52 / 11.6) compared to Sham group (P = 0.007). After treatment with HHL extract, apoptosis cells decreased significantly to 0.28% (0.025/0.3) compared to no-treated group and vitamin E treated group [3.8% (1.7/4.1)] (P = 0.02 < 0.05 and P = 0.04, respectively) (Figure 4).
DiscussionIt is well demonstrated that oxidative stress and excessive production of ROS play a major role in several aspects of ischemia and reperfusion.18 In this study, we evaluated the effect of HHL extract compared to vitamin E solution during hepatic I-R episodes.
In the first part, we focused on the quantification of HHL phenols and its antioxidant activity. In order to evaluate the antioxidant capacity of the samples, a method based on the reduction of DPPH was performed. DPPH radical is one of the few stable organic nitrogen free radicals, which is widely used to determine the free radical-scavenging ability of various samples.
The extract of HHL shows similar antioxidant capacity compared to vitamin E but the inhibition of lipid peroxidation damage (in vitro test) with plant extract was higher than the antioxidant reference. Therapeutic effects of plants are explained by antioxidant activities of phenols. In fact, the capacity of extracts to scavenger free radicals can be attributed to phenolic compounds.19 In our study, the high antioxidant effect of HHL is correlated to the high concentration in polyphenols.
Hepatic ischemia reperfusionDuring ischemic reperfusion periods, several functional changes occur at the cellular level promoting cell injury. A decrease in oxidative phosphorylation results in ATP depletion and derangements in calcium homeostasis.20 ROS have been implicated in the pathogenesis of I-R injury in the liver. Studies checking the sources of oxidant stress revealed that there is no evidence for a relevant in-tracellular oxidant stress in viable hepatocytes during reperfusion.21,22 Because of the potential inhibition of ROS by antioxidant agents, several studies tested pharma-cologic therapies in the modulation of the severity of I-R injury. Therefore, the administration of exogenous antioxidants, particularly in the early stages of reperfusion, could significantly decrease the severity of I-R damage in transplanted livers.
In this study, we evaluated the effect of phenols compounds in HHL extract compared to a reference antioxidant (vitamin E) during hepatic ischemia reperfusion episodes. Moreover, no studies have reported the effective role of Hypericum humifusum on liver I-R injury in rats. However, many studies showed that vitamin E had a protect effect against renal,23 pulmonary,24 retina25 and liver26–28 ischemia-reperfusion injury.
Lipid peroxidationROS generation play a major role in the occurrence of injury in cellular membranes leading to lipid peroxidation in liver ischemia reperfusion. In order to determine the extent of damage, MDA rate was used, as the final product of lipid peroxidation.1,29
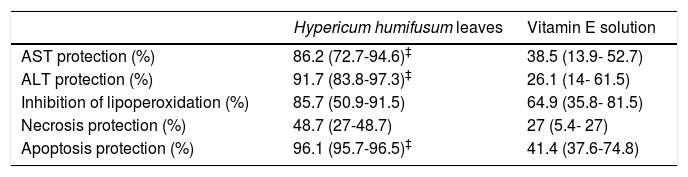
In the present study, the level of MDA, which is the product of lipid peroxidation, significantly increased by IR. This observation is in agreement with previous studies where elevated levels of lipid peroxidation products were detected.30,31 Treatment with Vitamin E decreased lipid peroxidation (64.9%) after I-R (Table 2). In fact, vitamin E neutralizes lipid peroxyl radicals, leading to the formation of tocopheroxyl radical, a relatively stable compound that alone cannot initiating the lipid peroxidation chain.32 HHL extract treatment decreases the production of lipid peroxidation (85.7%) more than vitamin E, but the difference between the two treated groups was not significant P = 0.2 (Table 2).
Percentage protections of Hypericum humifusum leaves and vitamin E solution against I-R liver injury.
| Hypericum humifusum leaves | Vitamin E solution | |
|---|---|---|
| AST protection (%) | 86.2 (72.7-94.6)‡ | 38.5 (13.9- 52.7) |
| ALT protection (%) | 91.7 (83.8-97.3)‡ | 26.1 (14- 61.5) |
| Inhibition of lipoperoxidation (%) | 85.7 (50.9-91.5) | 64.9 (35.8- 81.5) |
| Necrosis protection (%) | 48.7 (27-48.7) | 27 (5.4- 27) |
| Apoptosis protection (%) | 96.1 (95.7-96.5)‡ | 41.4 (37.6-74.8) |
Values are expressed as medians (min - max) (n = 5). Alanine Aminotransferase (ALT) and aspartate aminotransferase (AST). P < 0.05 ‡vs. treated vitamin E.
Literature reported that the species of Hypericum had a cytoprotective effect. It ameliorated free radical-induced reperfusion injury and modified the response pattern of several defense mechanisms. Benedi, et al.33 and Sanchez-Reus, et al.34 demonstrated that Hypericum perforatum reduce oxidative stress by inhibiting free radical generation and lipid peroxidation.
Bayramoglu, et al.35 demonstrated also that Hypericum perforatum increase the activities of glutathion peroxydase and catalase after hepatic I-R. In our study, oxidant and anti-oxidant enzymatic assays were not used.
The study of Elimadi, et al. demonstrate that oxidative stress induced by I-R was associated with increase in AST and ALT plasma levels.36
Enzymatic liverAST and ALT levels are considered as markers of hepa-tocellular injury and a good indicator of structural membrane damage.37 Thus, this test allows us to determine the ability of plant extract to protect liver against the damage effects of ischemia.38 Previous studies have shown that the serum concentrations of these enzymes increase in proportion with the duration of ischemia.39
In our study, we have found that the serum levels of AST and ALT increased after ischemia and reperfusion phases. In HHL treated group, the serum transaminases levels were significantly lower than those of the non-treated group. This suggests that HHL attenuates liver tissue damage induced by ischemia-reperfusion. HHL extract reduced significantly levels (P = 0.02) of AST and ALT activities (86.2% and 91.7%, respectively) compared to vitamin E solution (38.5% and 26.1%, respectively) (Table 2).
The increase of AST and ALT levels observed in I-R group can be explained by lipid peroxidation leading to cytolysis, which is caused by oxygen free radicals formed during the reperfusion phase.40 Increased levels of liver enzymes indicate hepatocyte destruction and this is in concordance with the histopathologic results.
Histopathological changesTo clarify the pathogenesis of ischemic liver injury, histopathological changes in the liver after ischemia reperfusion were examined in rats. I-R injury induces cell death through apoptosis and necrosis41 or a combination of both called necroapoptosis.42
Treatment with HHL at a dose of 25 mg/kg reduce significantly (P = 0.04) apoptotic cells (96.1%) more than vitamin E solution (41.4%). Inhibition of apoptosis cells seems to be a rational strategy to reduce liver injury induced by I-R. Moreover, many diseases can result from cells apoptotic formation like neurodegenerative diseases, hematologic diseases, and general tissue damage.43 So, HHL can be used against diseases related to the formation of apoptotic cells.
Immunohistochemistry study was necessary (Bcl-2, caspase 3…) to identify the molecular pathway responsible of the proliferation of apoptotic cells that can be protected by HHL.
Necrosis lesions persist in the two treated groups in HHL extract and vitamin E solution (48.7% and 27% respectively, P = 0.054) (Table 2).
In fact, we observed necrotic lesions at reversible phase for HHL treated group. While, necrosis areas at irreversible phases were observed in vitamin E treated group.
In conclusion, HHL extract protects hepatocytes from the deleterious effect induced by ischemia reperfusion in rat model. This cytoprotective effect is characterized by a reduction of the leakage of hepatic enzymes and MDA contents that attenuate liver injury.
HHL extract [86.2% (48.7/96.1)] protects significantly against ischemia-reperfusion damage more than vitamin E solution [38.5% (26.1 / 64.9)] (P = 0.04).
We suggest that HHL extract may be useful in the therapy of conditions associated with ischemia-reperfusion and damage related to apoptosis formation.
Abbreviations- •
ALT: alanine aminotransferase.
- •
AST: aspartate aminotransferase.
- •
ATP: adenosine triphosphate.
- •
DPPH: 2,2-diphenyl-1-picrylhydrazyl.
- •
EMIT: enzyme multiplied immunoassay technique.
- •
FC: folin-Ciocalteu.
- •
H&E: hematoxylin & eosin.
- •
HHL: hypericum humifusum leaves.
- •
HPF: high power fields.
- •
I-R: ischemia-reperfusion.
- •
MDA: Malondialdehyde.
- •
min / max: minimum / maximum.
- •
ROS: reactive oxygen species.
- •
SD: standard deviation.
- •
TBA: thiobarbituric acid.
- •
TM: trichrome of Masson.
Source of financial support in the form of grants: National Center of Pharmacovigilance & Laboratory of Clinical and Experimental Pharmacology LR16SP02.
AcknowledgmentThanks are due to Pr Mohamed Boussaid, Botanic Biotechnology Department of National Institute of Applied and Technology of Tunis.